当前位置:
X-MOL 学术
›
ACS ES&T Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Technoeconomic Assessment of Electrochemical Hydrogen Peroxide Production with Gas Diffusion Electrodes under Scenarios Relevant to Practical Water Treatment
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2023-08-23 , DOI: 10.1021/acsestengg.3c00238 Erzhuo Zhao 1 , Guangsen Xia 2 , Yang Li 1 , Juhong Zhan 1, 3 , Gang Yu 1 , Yujue Wang 1, 3
ACS ES&T Engineering ( IF 7.4 ) Pub Date : 2023-08-23 , DOI: 10.1021/acsestengg.3c00238 Erzhuo Zhao 1 , Guangsen Xia 2 , Yang Li 1 , Juhong Zhan 1, 3 , Gang Yu 1 , Yujue Wang 1, 3
Affiliation
|
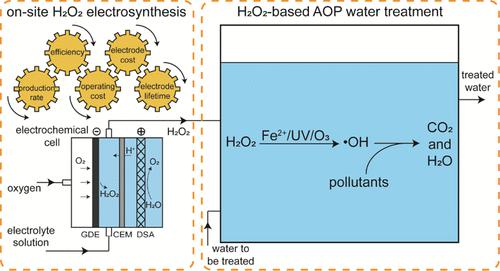
Recent studies have shown that electrochemically producing hydrogen peroxide (H2O2) with gas diffusion electrodes (GDEs) directly in the water to be treated by H2O2-based advanced oxidation processes (AOPs) is problematic in practical applications because of the quick deterioration of GDE stability by oxidation with strong oxidants (e.g., hydroxyl radicals) and fouling by complex water constituents (e.g., Ca2+ and Mg2+). Therefore, this study tested the electrosynthesis of H2O2 with GDEs in electrolyte solutions in a separate reactor as an alternative for on-site H2O2 production in water treatment. Results show that many reactor configurations and operational parameters (e.g., electrode distance, current densities, electrolytes, and GDE backpressure) intertwine to have complex influences on the overall performance of H2O2 production in terms of H2O2 production rates and current efficiencies, GDE stability, operating cost, and GDE capital cost. Under optimized conditions determined in this study (4 mm electrode distance, 150–200 mA/cm2 current densities, 1 M Na2SO4, and 30 kPa GDE backpressure), ∼29,000–34,000 mg/L of H2O2 could be produced with production rates of 57.3–68.3 mg/h/cm2 and apparent current efficiencies of 55%–61% during H2O2 electrosynthesis with a divided cell, and the GDEs maintained stable H2O2 production over ∼350–1000 h before water penetrated the GDEs due to the electrocapillary effect. The operating cost, including the electricity, electrolytes, water, and oxygen consumed in the process, was ∼1.32–1.48 $/kgH2O2, and the capital cost was ∼0.30–0.46 $/kgH2O2. The results of this study suggest that it is technoeconomically feasible to scale up H2O2 electrosynthesis with GDEs in electrolytes to produce H2O2 on site in some water treatment applications, e.g., micropollutant removal in drinking water treatment by H2O2-based AOPs. Additional studies are needed to further extend the GDE lifetime by improving GDE fabrications and operations to prevent water penetration during H2O2 electrosynthesis.
中文翻译:

实际水处理相关场景下气体扩散电极电化学生产过氧化氢的技术经济评估
最近的研究表明,直接在基于 H 2 O 2的高级氧化工艺(AOP )处理的水中用气体扩散电极(GDE)电化学生产过氧化氢(H 2 O 2)在实际应用中存在问题,因为强氧化剂(例如羟基自由基)的氧化和复杂水成分(例如Ca 2+和Mg 2+)的污染会导致GDE 稳定性快速恶化。因此,本研究测试了在单独的反应器中电解质溶液中用 GDE 电合成 H 2 O 2作为水处理中现场 H 2 O 2生产的替代方案。结果表明,许多反应器配置和操作参数(例如,电极距离、电流密度、电解质和 GDE 背压)相互交织,对 H 2 O 2生产的整体性能(在 H 2 O 2生产率和电流方面)产生复杂的影响。效率、GDE 稳定性、运营成本和 GDE 资本成本。在本研究确定的优化条件下(4 mm 电极距离、150–200 mA/cm 2电流密度、1 M Na 2 SO 4和 30 kPa GDE 背压),〜29,000–34,000 mg/L 的 H 2 O 2可以在使用分割池进行H 2 O 2电合成过程中,生产速率为 57.3–68.3 mg/h/cm 2 ,表观电流效率为 55%–61%,并且 GDE 在 ~350– 内保持稳定的 H 2 O 2产量。由于电毛细管效应,水渗透 GDE 之前 1000 小时。运营成本(包括过程中消耗的电力、电解质、水和氧气)为~1.32–1.48 美元/千克H2O2,资本成本为~0.30–0.46 美元/千克H2O2。本研究的结果表明,在一些水处理应用中,例如通过 H 2 O 2去除饮用水处理中的微污染物,在电解质中使用 GDE扩大H 2 O 2电合成规模以现场生产 H 2 O 2在技术经济上是可行的基于AOP。需要进行更多研究,通过改进 GDE 制造和操作来进一步延长 GDE 寿命,以防止 H 2 O 2电合成过程中的水渗透。
更新日期:2023-08-23
中文翻译:

实际水处理相关场景下气体扩散电极电化学生产过氧化氢的技术经济评估
最近的研究表明,直接在基于 H 2 O 2的高级氧化工艺(AOP )处理的水中用气体扩散电极(GDE)电化学生产过氧化氢(H 2 O 2)在实际应用中存在问题,因为强氧化剂(例如羟基自由基)的氧化和复杂水成分(例如Ca 2+和Mg 2+)的污染会导致GDE 稳定性快速恶化。因此,本研究测试了在单独的反应器中电解质溶液中用 GDE 电合成 H 2 O 2作为水处理中现场 H 2 O 2生产的替代方案。结果表明,许多反应器配置和操作参数(例如,电极距离、电流密度、电解质和 GDE 背压)相互交织,对 H 2 O 2生产的整体性能(在 H 2 O 2生产率和电流方面)产生复杂的影响。效率、GDE 稳定性、运营成本和 GDE 资本成本。在本研究确定的优化条件下(4 mm 电极距离、150–200 mA/cm 2电流密度、1 M Na 2 SO 4和 30 kPa GDE 背压),〜29,000–34,000 mg/L 的 H 2 O 2可以在使用分割池进行H 2 O 2电合成过程中,生产速率为 57.3–68.3 mg/h/cm 2 ,表观电流效率为 55%–61%,并且 GDE 在 ~350– 内保持稳定的 H 2 O 2产量。由于电毛细管效应,水渗透 GDE 之前 1000 小时。运营成本(包括过程中消耗的电力、电解质、水和氧气)为~1.32–1.48 美元/千克H2O2,资本成本为~0.30–0.46 美元/千克H2O2。本研究的结果表明,在一些水处理应用中,例如通过 H 2 O 2去除饮用水处理中的微污染物,在电解质中使用 GDE扩大H 2 O 2电合成规模以现场生产 H 2 O 2在技术经济上是可行的基于AOP。需要进行更多研究,通过改进 GDE 制造和操作来进一步延长 GDE 寿命,以防止 H 2 O 2电合成过程中的水渗透。

































 京公网安备 11010802027423号
京公网安备 11010802027423号