Separation and Purification Technology ( IF 8.1 ) Pub Date : 2023-08-22 , DOI: 10.1016/j.seppur.2023.124851 Chao Zheng , Kai Kang , Yucong Xie , Xuanlin Yang , Liang Lan , Hua Song , Hao Han , Shupei Bai
|
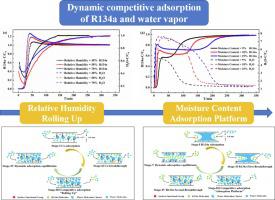
1.1.1.2-Tetrafluoroethane (R134a) is developed as a tracer for mechanical leakage detection in military chemistry. Due to the weaker physical adsorption properties, water vapor competitive adsorption limits the use of tracer R134a. Thus, it is urgent to the analyze dynamic competitive adsorption behavior of R134a and water vapor on the activated carbon. In this study, R134a breakthrough curves on the fixed beds packed with activated carbons under different relative humidity and moisture content levels were systematically measured. Combining mass spectrometry, gas chromatography, and a novel fiber Bragg grating temperature sensor, the dynamic competitive adsorption processes and axial temperature of fixed beds were first reported. Interestingly, with increasing moisture content, R134a breakthrough time decreased, but dynamic adsorption capacity increased. This was attributed to the fact that the residual water vapor could increase the specific surface area and pore volume of activated carbon to enhance R134a dynamic adsorption capacity. Using the R134a as the standard, the dynamic competitive adsorption process was first revealed and could be divided into four stages including Co-adsorption, Co-breakthrough, Competitive Adsorption (“Rolling Up” and “Adsorption Platform”), and Dynamic Adsorption Equilibrium. “Rolling Up” and “Adsorption Platform” phenomena are mainly determined by the strength of intermolecular interactions, the concentration difference of adsorbate, and the thermal effect induced by water vapor adsorption/desorption.
中文翻译:

不同湿度和含水量下1.1.1.2-四氟乙烷(R134a)在活性炭床上的动态吸附行为
1.1.1.2-四氟乙烷 (R134a) 是作为军事化学中机械泄漏检测的示踪剂而开发的。由于物理吸附性能较弱,水蒸气竞争吸附限制了示踪剂R134a的使用。因此,迫切需要分析R134a和水蒸气在活性炭上的动态竞争吸附行为。本研究系统地测量了不同相对湿度和含水量水平下填充活性炭的固定床上的R134a突破曲线。结合质谱、气相色谱和新型光纤布拉格光栅温度传感器,首次报道了固定床的动态竞争吸附过程和轴向温度。有趣的是,随着水分含量的增加,R134a 的突破时间减少,但动态吸附容量有所增加。这是由于残留的水蒸气可以增加活性炭的比表面积和孔体积,从而增强R134a的动态吸附能力。以R134a为标准,首次揭示了动态竞争吸附过程,可分为共吸附、共突破、竞争吸附(“卷起”和“吸附平台”)和动态吸附平衡四个阶段。“卷起”和“吸附平台”现象主要由分子间相互作用的强度、吸附质的浓度差以及水蒸气吸附/解吸引起的热效应决定。这是由于残留的水蒸气可以增加活性炭的比表面积和孔体积,从而增强R134a的动态吸附能力。以R134a为标准,首次揭示了动态竞争吸附过程,可分为共吸附、共突破、竞争吸附(“卷起”和“吸附平台”)和动态吸附平衡四个阶段。“卷起”和“吸附平台”现象主要由分子间相互作用的强度、吸附质的浓度差以及水蒸气吸附/解吸引起的热效应决定。这是由于残留的水蒸气可以增加活性炭的比表面积和孔体积,从而增强R134a的动态吸附能力。以R134a为标准,首次揭示了动态竞争吸附过程,可分为共吸附、共突破、竞争吸附(“卷起”和“吸附平台”)和动态吸附平衡四个阶段。“卷起”和“吸附平台”现象主要由分子间相互作用的强度、吸附质的浓度差以及水蒸气吸附/解吸引起的热效应决定。首次揭示了动态竞争吸附过程,可分为共吸附、共突破、竞争吸附(“卷起”和“吸附平台”)和动态吸附平衡四个阶段。“卷起”和“吸附平台”现象主要由分子间相互作用的强度、吸附质的浓度差以及水蒸气吸附/解吸引起的热效应决定。首次揭示了动态竞争吸附过程,可分为共吸附、共突破、竞争吸附(“卷起”和“吸附平台”)和动态吸附平衡四个阶段。“卷起”和“吸附平台”现象主要由分子间相互作用的强度、吸附质的浓度差以及水蒸气吸附/解吸引起的热效应决定。

































 京公网安备 11010802027423号
京公网安备 11010802027423号