当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Highly Selective and Orally Bioavailable PI3Kδ Inhibitors with Anti-Inflammatory Activity for Treatment of Acute Lung Injury
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-08-22 , DOI: 10.1021/acs.jmedchem.3c00508
Yongmei Tang 1 , Fanli Zheng 2 , Xiaodong Bao 1 , Yanan Zheng 2 , Xueping Hu 3 , Siyue Lou 2 , Huajun Zhao 2 , Sunliang Cui 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-08-22 , DOI: 10.1021/acs.jmedchem.3c00508
Yongmei Tang 1 , Fanli Zheng 2 , Xiaodong Bao 1 , Yanan Zheng 2 , Xueping Hu 3 , Siyue Lou 2 , Huajun Zhao 2 , Sunliang Cui 1
Affiliation
|
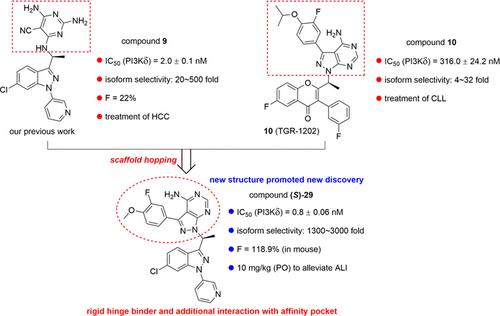
PI3Kδ is a promising target for the treatment of inflammatory disease; however, the application of PI3Kδ inhibitors in acute respiratory inflammatory diseases is rarely investigated. In this study, through scaffold hopping design, we report a new series of 1H-pyrazolo[3,4-d]pyrimidin-4-amine-tethered 3-methyl-1-aryl-1H-indazoles as highly selective and potent PI3Kδ inhibitors with significant anti-inflammatory activities for treatment of acute lung injury (ALI). There were 29 compounds designed, prepared, and subjected to PI3Kδ inhibitory activity evaluation and anti-inflammatory activity evaluation in macrophages. (S)-29 was identified as a candidate with high PI3Kδ inhibitory activity, isoform selectivity, and high oral bioavailability. The in vivo administration of (S)-29 at 10 mg/kg dosage could significantly ameliorate histopathological changes and attenuate lung inflammation in lung tissues of LPS-challenged mice. Molecular docking demonstrated the success of scaffold hopping design. Overall, (S)-29 is a potent PI3Kδ inhibitor which might be a promising candidate for the treatment of ALI.
中文翻译:

发现具有抗炎活性的高选择性、口服生物可利用的 PI3Kδ 抑制剂,用于治疗急性肺损伤
PI3Kδ是治疗炎症性疾病的一个有前景的靶点;然而,PI3Kδ抑制剂在急性呼吸道炎症性疾病中的应用却鲜有研究。在本研究中,通过支架跳跃设计,我们报告了一系列新的 1 H -吡唑并[3,4- d ]嘧啶-4-胺系链 3-甲基-1-芳基-1 H -吲唑,具有高选择性和有效的特性PI3Kδ 抑制剂具有显着的抗炎活性,可用于治疗急性肺损伤(ALI)。设计、制备了29种化合物,并在巨噬细胞中进行了PI3Kδ抑制活性评价和抗炎活性评价。 ( S )-29被确定为具有高 PI3Kδ 抑制活性、亚型选择性和高口服生物利用度的候选药物。体内施用10 mg/kg剂量的( S )-29可以显着改善LPS攻击小鼠肺组织的组织病理学变化并减轻肺部炎症。分子对接证明了支架跳跃设计的成功。总体而言, ( S )-29是一种有效的 PI3Kδ 抑制剂,可能是治疗 ALI 的有希望的候选药物。
更新日期:2023-08-22
中文翻译:

发现具有抗炎活性的高选择性、口服生物可利用的 PI3Kδ 抑制剂,用于治疗急性肺损伤
PI3Kδ是治疗炎症性疾病的一个有前景的靶点;然而,PI3Kδ抑制剂在急性呼吸道炎症性疾病中的应用却鲜有研究。在本研究中,通过支架跳跃设计,我们报告了一系列新的 1 H -吡唑并[3,4- d ]嘧啶-4-胺系链 3-甲基-1-芳基-1 H -吲唑,具有高选择性和有效的特性PI3Kδ 抑制剂具有显着的抗炎活性,可用于治疗急性肺损伤(ALI)。设计、制备了29种化合物,并在巨噬细胞中进行了PI3Kδ抑制活性评价和抗炎活性评价。 ( S )-29被确定为具有高 PI3Kδ 抑制活性、亚型选择性和高口服生物利用度的候选药物。体内施用10 mg/kg剂量的( S )-29可以显着改善LPS攻击小鼠肺组织的组织病理学变化并减轻肺部炎症。分子对接证明了支架跳跃设计的成功。总体而言, ( S )-29是一种有效的 PI3Kδ 抑制剂,可能是治疗 ALI 的有希望的候选药物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号