当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Macroporous Carbon-Nitride-Supported Transition-Metal Single-Atom Catalysts for Photocatalytic Hydrogen Production from Ammonia Splitting
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-08-21 , DOI: 10.1021/acscatal.3c02076 Jingkai Lin 1 , Yantao Wang 1, 2 , Wenjie Tian 1 , Huayang Zhang 1 , Hongqi Sun 3 , Shaobin Wang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-08-21 , DOI: 10.1021/acscatal.3c02076 Jingkai Lin 1 , Yantao Wang 1, 2 , Wenjie Tian 1 , Huayang Zhang 1 , Hongqi Sun 3 , Shaobin Wang 1
Affiliation
|
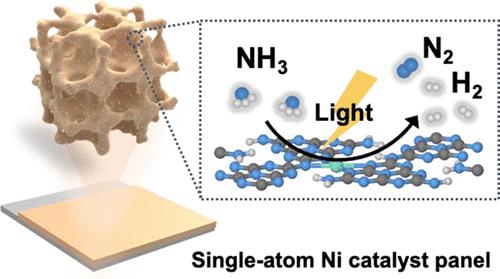
Ammonia (NH3) splitting to hydrogen (H2) is a promising route for on-site production of green hydrogen energy; however, the application is limited due to high-cost noble-metal-based catalysts and high operating temperature of the endothermic nature. Herein, we develop a series of macroporous carbon nitride-supported single-atom transition metal (TMs-MCN, TMs: Co, Mn, Fe, Ni, Cu) catalyst panels for solar light-driven photocatalytic gaseous NH3 splitting. Under ambient reaction conditions, the optimized Ni-MCN shows an H2 production rate of 35.6 μmol g–1 h–1, much superior to that of MCN and other TMs-MCN. Such enhanced photoactivity is attributed to the presence of Ni–N4 sites, which improve the optical properties, accelerate charge carrier separation/transfer, and boost NH3 splitting kinetics of the catalysts. Density functional theory calculations further reveal that the Ni–N4 sites can effectively modify the electronic structure of the carbon nitride. Compared with other metal sites, the Ni–N4 site possesses moderate NH3 binding strength and the lowest energy barrier to facilitate the formation of key intermediates *NH + *H. These findings provide valuable guidelines for the rational design of single-atom catalysts toward energy- and cost-effective photocatalytic NH3 splitting for H2 production.
中文翻译:

大孔氮化碳负载过渡金属单原子催化剂用于光催化氨分解制氢
氨(NH 3)分解为氢气(H 2)是现场生产绿色氢能的一条有前途的途径;然而,由于贵金属基催化剂成本高且吸热性质操作温度高,其应用受到限制。在此,我们开发了一系列大孔氮化碳负载的单原子过渡金属(TMs-MCN,TMs:Co、Mn、Fe、Ni、Cu)催化剂板,用于太阳能光驱动的光催化气态NH 3分解。在常温反应条件下,优化后的Ni-MCN的H 2产率为35.6 μmol g –1 h –1,远优于MCN和其他TMs-MCN。这种增强的光活性归因于 Ni-N 的存在4 个位点,可改善光学性能,加速载流子分离/转移,并增强催化剂的NH 3分解动力学。密度泛函理论计算进一步表明Ni-N 4位点可以有效改变氮化碳的电子结构。与其他金属位点相比,Ni-N 4位点具有中等的NH 3结合强度和最低的能垒,有利于关键中间体*NH + *H的形成。这些发现为单原子催化剂的合理设计提供了有价值的指导,以实现能源和成本效益高的光催化NH 3分解生产H 2。
更新日期:2023-08-21
中文翻译:

大孔氮化碳负载过渡金属单原子催化剂用于光催化氨分解制氢
氨(NH 3)分解为氢气(H 2)是现场生产绿色氢能的一条有前途的途径;然而,由于贵金属基催化剂成本高且吸热性质操作温度高,其应用受到限制。在此,我们开发了一系列大孔氮化碳负载的单原子过渡金属(TMs-MCN,TMs:Co、Mn、Fe、Ni、Cu)催化剂板,用于太阳能光驱动的光催化气态NH 3分解。在常温反应条件下,优化后的Ni-MCN的H 2产率为35.6 μmol g –1 h –1,远优于MCN和其他TMs-MCN。这种增强的光活性归因于 Ni-N 的存在4 个位点,可改善光学性能,加速载流子分离/转移,并增强催化剂的NH 3分解动力学。密度泛函理论计算进一步表明Ni-N 4位点可以有效改变氮化碳的电子结构。与其他金属位点相比,Ni-N 4位点具有中等的NH 3结合强度和最低的能垒,有利于关键中间体*NH + *H的形成。这些发现为单原子催化剂的合理设计提供了有价值的指导,以实现能源和成本效益高的光催化NH 3分解生产H 2。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号