当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, in Silico Study and Biological Evaluation of N-(Benzothiazol/Thiazol-2-yl)benzamide Derivatives as Quorum Sensing Inhibitors against Pseudomonas aeruginosa
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-08-21 , DOI: 10.1002/cbdv.202300647 Nikhil Sharma 1 , Namita Srivastava 2 , Ashutosh Kaushal 1 , Bhanuranjan Das 3 , Aditi Vashistha 1 , Lokender Kumar 2, 4 , Rajnish Kumar 3 , Ashok Kumar Yadav 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2023-08-21 , DOI: 10.1002/cbdv.202300647 Nikhil Sharma 1 , Namita Srivastava 2 , Ashutosh Kaushal 1 , Bhanuranjan Das 3 , Aditi Vashistha 1 , Lokender Kumar 2, 4 , Rajnish Kumar 3 , Ashok Kumar Yadav 1
Affiliation
|
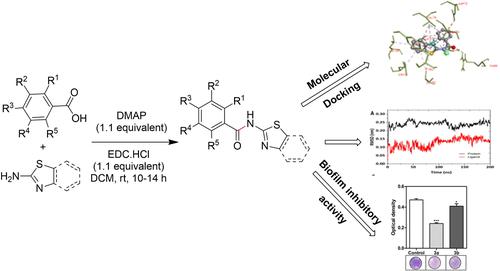
The development of bacterial resistance to chemical therapy poses a severe danger to efficacy of treating bacterial infections. One of the key factors for resistance to antimicrobial medications is growth of bacteria in biofilm. Quorum sensing (QS) inhibition was created as an alternative treatment by developing novel anti-biofilm medicines. Cell-cell communication is impeded by QS inhibition, which targets QS signaling pathway. The goal of this work is to develop newer drugs that are effective against Pseudomonas aeruginosa by decreasing QS and acting as anti-biofilm agents. In this investigation, N-(benzo[d]thiazol-2-yl)benzamide/N-(thiazol-2-yl)benzamide derivatives 3a-h were designed and synthesized in good yields. Further, molecular docking analyses revealed that binding affinity values were founded −11.2 to −7.6 kcal/mol that were moderate to good. The physicochemical properties of these prepared compounds were investigated through in-silico method. Molecular dynamic simulation was also used to know better understanding of stability of the protein and ligand complex. Comparing N-(benzo[d]thiazol-2-yl)benzamide 3a to salicylic acid (4.40±0.10) that was utilised as standard for quorum sensing inhibitor, the anti-QS action was found greater for N-(benzo[d]thiazol-2-yl)benzamide 3a (4.67±0.45) than salicylic acid (4.40±0.10). Overall, research results suggested that N-(benzo[d]thiazol-2-yl)benzamide/N-(thiazol-2-yl)benzamide derivatives 3a-h may hold to develop new quorum sensing inhibitors.
中文翻译:

N-(苯并噻唑/噻唑-2-基)苯甲酰胺衍生物作为铜绿假单胞菌群体感应抑制剂的合成、计算机研究和生物学评价
细菌对化学疗法产生耐药性对治疗细菌感染的功效构成严重危险。抗菌药物耐药性的关键因素之一是生物膜中细菌的生长。通过开发新型抗生物膜药物,群体感应(QS)抑制作为一种替代治疗方法而诞生。QS 抑制作用是针对 QS 信号通路的,从而阻碍细胞间的通讯。这项工作的目标是开发通过降低 QS 并充当抗生物膜剂来有效对抗铜绿假单胞菌的新药物。在本研究中,设计并以良好的产率合成了N- (苯并[ d ]噻唑-2-基)苯甲酰胺/ N- (噻唑-2-基)苯甲酰胺衍生物3a-h 。此外,分子对接分析显示,结合亲和力值为-11.2至-7.6 kcal/mol,中等至良好。通过计算机模拟方法研究了这些制备的化合物的物理化学性质。分子动力学模拟也用于更好地了解蛋白质和配体复合物的稳定性。将N -(苯并[ d ]噻唑-2-基)苯甲酰胺3a与用作群体感应抑制剂标准品的水杨酸 (4.40±0.10)进行比较,发现N -(苯并[ d ])的抗 QS 作用更大噻唑-2-基)苯甲酰胺3a (4.67±0.45) 高于水杨酸 (4.40±0.10)。总体而言,研究结果表明N- (苯并[ d ]噻唑-2-基)苯甲酰胺/ N- (噻唑-2-基)苯甲酰胺衍生物3a-h可能适合开发新的群体感应抑制剂。
更新日期:2023-08-21
中文翻译:

N-(苯并噻唑/噻唑-2-基)苯甲酰胺衍生物作为铜绿假单胞菌群体感应抑制剂的合成、计算机研究和生物学评价
细菌对化学疗法产生耐药性对治疗细菌感染的功效构成严重危险。抗菌药物耐药性的关键因素之一是生物膜中细菌的生长。通过开发新型抗生物膜药物,群体感应(QS)抑制作为一种替代治疗方法而诞生。QS 抑制作用是针对 QS 信号通路的,从而阻碍细胞间的通讯。这项工作的目标是开发通过降低 QS 并充当抗生物膜剂来有效对抗铜绿假单胞菌的新药物。在本研究中,设计并以良好的产率合成了N- (苯并[ d ]噻唑-2-基)苯甲酰胺/ N- (噻唑-2-基)苯甲酰胺衍生物3a-h 。此外,分子对接分析显示,结合亲和力值为-11.2至-7.6 kcal/mol,中等至良好。通过计算机模拟方法研究了这些制备的化合物的物理化学性质。分子动力学模拟也用于更好地了解蛋白质和配体复合物的稳定性。将N -(苯并[ d ]噻唑-2-基)苯甲酰胺3a与用作群体感应抑制剂标准品的水杨酸 (4.40±0.10)进行比较,发现N -(苯并[ d ])的抗 QS 作用更大噻唑-2-基)苯甲酰胺3a (4.67±0.45) 高于水杨酸 (4.40±0.10)。总体而言,研究结果表明N- (苯并[ d ]噻唑-2-基)苯甲酰胺/ N- (噻唑-2-基)苯甲酰胺衍生物3a-h可能适合开发新的群体感应抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号