Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Positive Chemotaxis of CREKA-Modified Ceria@Polydopamine Biomimetic Nanoswimmers for Enhanced Penetration and Chemo-photothermal Tumor Therapy
ACS Nano ( IF 15.8 ) Pub Date : 2023-08-18 , DOI: 10.1021/acsnano.3c05232 Minxia Zhu 1 , Luwen Zhu 1 , Yuchan You 1 , Mingchen Sun 1 , Feiyang Jin 1 , Yanling Song 1 , Jucong Zhang 1 , Xiaoling Xu 2 , Jiansong Ji 3 , Yongzhong Du 1
ACS Nano ( IF 15.8 ) Pub Date : 2023-08-18 , DOI: 10.1021/acsnano.3c05232 Minxia Zhu 1 , Luwen Zhu 1 , Yuchan You 1 , Mingchen Sun 1 , Feiyang Jin 1 , Yanling Song 1 , Jucong Zhang 1 , Xiaoling Xu 2 , Jiansong Ji 3 , Yongzhong Du 1
Affiliation
|
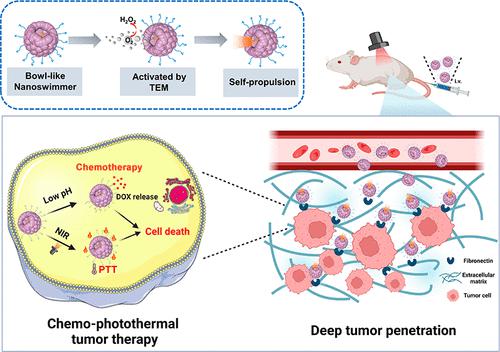
Tumor interstitial pressure represents the greatest barrier against drug diffusion into the depth of the tumor. Biometric nanomotors highlight the possibility of enhanced deep penetration and improve cellular uptake. However, control of their directionality remains difficult to achieve. Herein, we report cysteine-arginine-glutamic acid-lysine-alanine (CREKA)-modified ceria@polydopamine nanobowls as tumor microenvironment-fueled nanoscale motors for positive chemotaxis into the tumor depth or toward tumor cells. Upon laser irradiation, this nanoswimmer rapidly depletes the tumor microenvironment-specific hydrogen peroxide (H2O2) in the nanobowl, contributing to a self-generated gradient and subsequently propulsion (9.5 μm/s at 46 °C). Moreover, the asymmetrical modification of CREKA on nanobowls could automatically reconfigure the motion direction toward tumor depth or tumor cells in response to receptor–ligand interaction, leading to a deep penetration (70 μm in multicellular spheroids) and enhanced antitumor effects over conventional nanomedicine-induced chemo-photothermal therapy (tumor growth inhibition rate: 84.2% versus 56.9%). Thus, controlling the direction of nanomotors holds considerable potential for improved antitumor responses, especially in solid tumors with high tumor interstitial pressure.
中文翻译:

CREKA 修饰的 Ceria@Polydopamine 仿生 Nanoswimmers 的正趋化性,用于增强渗透和化学光热肿瘤治疗
肿瘤间质压力是阻止药物扩散到肿瘤深处的最大障碍。生物识别纳米马达强调了增强深度渗透和改善细胞吸收的可能性。然而,对其方向性的控制仍然难以实现。在此,我们报告半胱氨酸-精氨酸-谷氨酸-赖氨酸-丙氨酸(CREKA)修饰的二氧化铈@聚多巴胺纳米碗作为肿瘤微环境驱动的纳米级马达,用于向肿瘤深度或向肿瘤细胞正向趋化。在激光照射下,这种纳米游泳器迅速耗尽纳米碗中肿瘤微环境特异性的过氧化氢 (H 2 O 2 ),从而产生自生成的梯度和随后的推进力(46 °C 下为 9.5 μm/s)。此外,CREKA在纳米碗上的不对称修饰可以响应受体-配体相互作用自动重新配置朝向肿瘤深度或肿瘤细胞的运动方向,从而导致深度渗透(在多细胞球体中为70μm)并比传统纳米药物诱导的抗肿瘤效果增强化学光热疗法(肿瘤生长抑制率:84.2% vs 56.9%)。因此,控制纳米马达的方向对于改善抗肿瘤反应具有相当大的潜力,特别是在具有高肿瘤间质压力的实体瘤中。
更新日期:2023-08-18
中文翻译:

CREKA 修饰的 Ceria@Polydopamine 仿生 Nanoswimmers 的正趋化性,用于增强渗透和化学光热肿瘤治疗
肿瘤间质压力是阻止药物扩散到肿瘤深处的最大障碍。生物识别纳米马达强调了增强深度渗透和改善细胞吸收的可能性。然而,对其方向性的控制仍然难以实现。在此,我们报告半胱氨酸-精氨酸-谷氨酸-赖氨酸-丙氨酸(CREKA)修饰的二氧化铈@聚多巴胺纳米碗作为肿瘤微环境驱动的纳米级马达,用于向肿瘤深度或向肿瘤细胞正向趋化。在激光照射下,这种纳米游泳器迅速耗尽纳米碗中肿瘤微环境特异性的过氧化氢 (H 2 O 2 ),从而产生自生成的梯度和随后的推进力(46 °C 下为 9.5 μm/s)。此外,CREKA在纳米碗上的不对称修饰可以响应受体-配体相互作用自动重新配置朝向肿瘤深度或肿瘤细胞的运动方向,从而导致深度渗透(在多细胞球体中为70μm)并比传统纳米药物诱导的抗肿瘤效果增强化学光热疗法(肿瘤生长抑制率:84.2% vs 56.9%)。因此,控制纳米马达的方向对于改善抗肿瘤反应具有相当大的潜力,特别是在具有高肿瘤间质压力的实体瘤中。































 京公网安备 11010802027423号
京公网安备 11010802027423号