当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multiscale Simulation and Mechanism Analysis of Cyclohexane/Ethanol Separation by Ionic Liquid Extractive Distillation
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2023-08-15 , DOI: 10.1021/acs.iecr.3c00867 Xuehui Zhang 1 , Qingrui Zhang 1 , Kang Liu 1 , Siyuan Zhang 1 , Shuning Jiang 1 , Yanchao Xue 1 , Xinshun Tan 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2023-08-15 , DOI: 10.1021/acs.iecr.3c00867 Xuehui Zhang 1 , Qingrui Zhang 1 , Kang Liu 1 , Siyuan Zhang 1 , Shuning Jiang 1 , Yanchao Xue 1 , Xinshun Tan 1
Affiliation
|
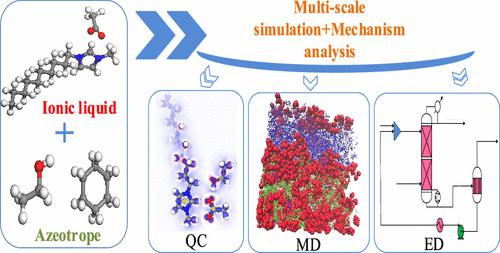
Ionic liquids (ILs) excel in extractive distillation (ED) for the separation of binary azeotropes due to their unique physical and chemical properties. However, the mechanism of action of ILs in azeotropes is unclear, and the present-day mechanistic studies have been conducted at specific concentrations. In this work, the formation and separation process of azeotropes and the phase behavior of each component at different component concentrations were studied microscopically by quantum chemistry (QC) calculations, molecular dynamics (MD) simulation, and process simulation. The hydrogen-bond donor–acceptor connection between solvents was outlined using the COSMO-SAC model. By using QC to evaluate the hydrogen-bond interaction between ethanol and ILs, the optimal extractant [Dmim][Ac] was chosen. The phase behavior of different concentrations of ILs with ethanol and cyclohexane was further investigated by MD. The two-dimensional atomic number density distribution (ANDD) instinctively indicates the mixing behavior and structural distribution of the cyclohexane–ethanol–ILs ternary system at the atomic level. The radial distribution function (RDF) and spatial distribution function (SDF) showed that the interaction between anions and ethanol in ILs was the strongest, and the amount of ILs had a significant impact on the cations throughout the separation procedure. Although anions have a strong attraction to cyclohexane and ethanol, this effect is not sensitive to the concentration of ILs. Aspen Plus V12 was used to simulate the separation of the cyclohexane–ethanol azeotropic system in the industrial process, and the feasibility of ILs as an extractant was verified. This work provides theoretical guidance for the study of the ED mechanism and solvent selection.
中文翻译:

离子液体萃取精馏分离环己烷/乙醇的多尺度模拟及机理分析
离子液体 (IL) 由于其独特的物理和化学性质,在萃取蒸馏 (ED) 分离二元共沸物方面表现出色。然而,离子液体在共沸物中的作用机制尚不清楚,目前的机理研究都是在特定浓度下进行的。本工作通过量子化学(QC)计算、分子动力学(MD)模拟和过程模拟,从微观上研究了共沸物的形成和分离过程以及不同组分浓度下各组分的相行为。使用 COSMO-SAC 模型概述了溶剂之间的氢键供体-受体连接。通过使用 QC 评估乙醇和 IL 之间的氢键相互作用,选择最佳提取剂 [Dmim][Ac]。通过 MD 进一步研究了不同浓度的离子液体与乙醇和环己烷的相行为。二维原子序数密度分布(ANDD)直观地表明了环己烷-乙醇-ILs三元体系在原子水平上的混合行为和结构分布。径向分布函数(RDF)和空间分布函数(SDF)表明,离子液体中阴离子与乙醇之间的相互作用最强,并且离子液体的量在整个分离过程中对阳离子有显着影响。虽然阴离子对环己烷和乙醇有很强的吸引力,但这种作用对离子液体的浓度不敏感。采用Aspen Plus V12模拟工业过程中环己烷-乙醇共沸体系的分离,验证了离子液体作为萃取剂的可行性。该工作为ED机理和溶剂选择的研究提供了理论指导。
更新日期:2023-08-15
中文翻译:

离子液体萃取精馏分离环己烷/乙醇的多尺度模拟及机理分析
离子液体 (IL) 由于其独特的物理和化学性质,在萃取蒸馏 (ED) 分离二元共沸物方面表现出色。然而,离子液体在共沸物中的作用机制尚不清楚,目前的机理研究都是在特定浓度下进行的。本工作通过量子化学(QC)计算、分子动力学(MD)模拟和过程模拟,从微观上研究了共沸物的形成和分离过程以及不同组分浓度下各组分的相行为。使用 COSMO-SAC 模型概述了溶剂之间的氢键供体-受体连接。通过使用 QC 评估乙醇和 IL 之间的氢键相互作用,选择最佳提取剂 [Dmim][Ac]。通过 MD 进一步研究了不同浓度的离子液体与乙醇和环己烷的相行为。二维原子序数密度分布(ANDD)直观地表明了环己烷-乙醇-ILs三元体系在原子水平上的混合行为和结构分布。径向分布函数(RDF)和空间分布函数(SDF)表明,离子液体中阴离子与乙醇之间的相互作用最强,并且离子液体的量在整个分离过程中对阳离子有显着影响。虽然阴离子对环己烷和乙醇有很强的吸引力,但这种作用对离子液体的浓度不敏感。采用Aspen Plus V12模拟工业过程中环己烷-乙醇共沸体系的分离,验证了离子液体作为萃取剂的可行性。该工作为ED机理和溶剂选择的研究提供了理论指导。










































 京公网安备 11010802027423号
京公网安备 11010802027423号