当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermal Atomic Layer Etching of Zinc Sulfide Using Sequential Trimethylaluminum and Hydrogen Fluoride Exposures: Evidence for a Conversion Mechanism
Chemistry of Materials ( IF 7.2 ) Pub Date : 2023-08-16 , DOI: 10.1021/acs.chemmater.3c00616 Taewook Nam 1 , Jonathan L. Partridge 1 , Steven M. George 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2023-08-16 , DOI: 10.1021/acs.chemmater.3c00616 Taewook Nam 1 , Jonathan L. Partridge 1 , Steven M. George 1
Affiliation
|
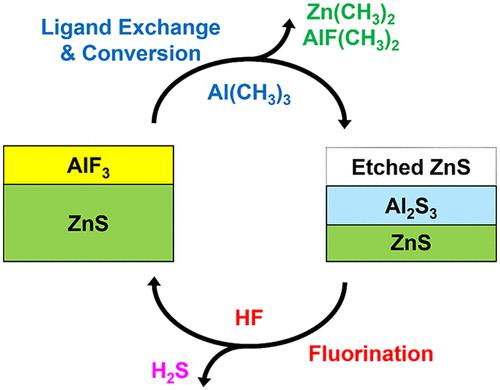
Thermal atomic layer etching (ALE) of zinc sulfide (ZnS) was demonstrated using sequential exposures of Al(CH3)3 (trimethylaluminum (TMA)) and HF (hydrogen fluoride). ZnS is one of the first sulfide materials to be etched using thermal ALE techniques. In situ spectroscopic ellipsometry (SE) studies were performed on ZnS films grown at 100 °C using atomic layer deposition (ALD) techniques. These studies revealed that the etch rate during ZnS ALE increased with temperature from 1.4 Å/cycle at 225 °C to 2.1 Å/cycle at 300 °C. ZnS ALE was also self-limiting at longer TMA and HF exposures. A possible mechanism for ZnS ALE is fluorination and ligand exchange where ZnS is fluorinated by HF and then ZnF2 undergoes ligand exchange with Al(CH3)3 to yield Zn(CH3)2. Because Al(CH3)3 may also have the ability to convert ZnS to Al2S3, a second possible mechanism for ZnS ALE is ligand exchange/conversion by TMA together with fluorination by HF. To verify the conversion mechanism, in situ quadruple mass spectrometry (QMS) studies revealed that Al(CH3)3 exposures on initial ZnS substrates released Zn(CH3)2 products, as expected for a conversion reaction. In addition, no H2S products were observed by QMS analysis during HF exposure on the initial ZnS substrates. However, after Al(CH3)3 exposures on ZnS, QMS measurements monitored H2S from HF exposures, as expected if Al(CH3)3 converts ZnS to Al2S3. These QMS results provide direct evidence for the conversion of ZnS to Al2S3 during ZnS ALE. Time-dependent QMS results also revealed that the conversion/ligand-exchange and fluorination reactions were self-limiting. In addition, QMS analysis observed AlxFy(CH3)z dimers and trimers as ligand-exchange products during the Al(CH3)3 exposures. Because the Al2S3 conversion layer thickness is dependent on Al(CH3)3 exposures, larger Al(CH3)3 pressures over equivalent times led to higher ZnS etch rates. In contrast, larger HF pressures over equivalent times had a small effect on the ZnS etch rate because HF is able to fluorinate only the converted Al2S3 layer thickness. The ZnS etch rate was slightly dependent on the ZnS ALD growth temperature. The ZnS etch rate at 300 °C was 1.4, 1.0, and 0.8 Å/cycle for ZnS ALD films grown at 100, 200, and 300 °C, respectively. The lower ZnS etch rates for the ZnS ALD films grown at higher temperatures were attributed to the larger density and higher sulfur content of ZnS ALD films grown at higher temperatures. The ZnS ALD films with a larger density may be more difficult to convert from ZnS to Al2S3 during the Al(CH3)3 reaction. The RMS roughness of the ZnS films was slightly decreased from 7.4 to 5.9 Å after 15 ALE cycles. However, after 30 ALE cycles, the RMS roughness gradually increased with the ALE cycles.
中文翻译:

使用连续三甲基铝和氟化氢暴露对硫化锌进行热原子层蚀刻:转换机制的证据
使用 Al(CH 3 ) 3(三甲基铝 (TMA))和 HF(氟化氢)的连续曝光演示了硫化锌 (ZnS) 的热原子层蚀刻 (ALE) 。ZnS 是最早使用热 ALE 技术蚀刻的硫化物材料之一。使用原子层沉积 (ALD) 技术对 100 °C 下生长的 ZnS 薄膜进行原位光谱椭圆光度 (SE) 研究。这些研究表明,ZnS ALE 期间的蚀刻速率随着温度的升高而增加,从 225 °C 时的 1.4 Å/循环增加到 300 °C 时的 2.1 Å/循环。ZnS ALE 在较长时间的 TMA 和 HF 暴露下也具有自限性。ZnS ALE 的一个可能机制是氟化和配体交换,其中 ZnS 通过 HF 氟化,然后 ZnF 2与 Al(CH 3) 3得到Zn(CH 3 ) 2。因为Al(CH 3 ) 3也可能具有将ZnS转化为Al 2 S 3的能力,所以ZnS ALE的第二种可能的机制是通过TMA的配体交换/转化以及通过HF的氟化。为了验证转化机制,原位四重质谱 (QMS) 研究表明,初始 ZnS 基底上的Al(CH 3 ) 3暴露释放了 Zn(CH 3 ) 2产物,正如转化反应所预期的那样。另外,没有H 2在初始 ZnS 基底上的 HF 暴露过程中,通过 QMS 分析观察到 S 产物。然而,在Al(CH 3 ) 3暴露于ZnS之后,QMS测量监测了HF暴露中的H 2 S,正如预期的,如果Al(CH 3 ) 3将ZnS转化为Al 2 S 3。这些 QMS 结果为 ZnS ALE 期间 ZnS 转化为 Al 2 S 3提供了直接证据。时间依赖性 QMS 结果还表明,转化/配体交换和氟化反应是自限性的。另外,QMS分析观察到Al x F y (CH 3 ) z在 Al(CH 3 ) 3暴露期间作为配体交换产物的二聚体和三聚体。因为Al 2 S 3转化层厚度取决于Al(CH 3 ) 3暴露,所以在同等时间内较大的Al(CH 3 ) 3压力导致较高的ZnS蚀刻速率。相反,在同等时间内较大的 HF 压力对 ZnS 蚀刻速率影响较小,因为 HF 只能氟化转化的 Al 2 S 3层厚度。ZnS 蚀刻速率稍微依赖于 ZnS ALD 生长温度。对于在 100、200 和 300 °C 生长的 ZnS ALD 薄膜,300 °C 下的 ZnS 蚀刻速率分别为 1.4、1.0 和 0.8 Å/周期。在较高温度下生长的 ZnS ALD 薄膜的较低 ZnS 蚀刻速率归因于在较高温度下生长的 ZnS ALD 薄膜具有较大的密度和较高的硫含量。具有较大密度的ZnS ALD膜在Al(CH 3 ) 3反应期间可能更难以从ZnS转化为Al 2 S 3。经过 15 次 ALE 循环后,ZnS 薄膜的 RMS 粗糙度从 7.4 埃略微下降至 5.9 埃。然而,在 30 个 ALE 循环后,RMS 粗糙度随着 ALE 循环逐渐增加。
更新日期:2023-08-16
中文翻译:

使用连续三甲基铝和氟化氢暴露对硫化锌进行热原子层蚀刻:转换机制的证据
使用 Al(CH 3 ) 3(三甲基铝 (TMA))和 HF(氟化氢)的连续曝光演示了硫化锌 (ZnS) 的热原子层蚀刻 (ALE) 。ZnS 是最早使用热 ALE 技术蚀刻的硫化物材料之一。使用原子层沉积 (ALD) 技术对 100 °C 下生长的 ZnS 薄膜进行原位光谱椭圆光度 (SE) 研究。这些研究表明,ZnS ALE 期间的蚀刻速率随着温度的升高而增加,从 225 °C 时的 1.4 Å/循环增加到 300 °C 时的 2.1 Å/循环。ZnS ALE 在较长时间的 TMA 和 HF 暴露下也具有自限性。ZnS ALE 的一个可能机制是氟化和配体交换,其中 ZnS 通过 HF 氟化,然后 ZnF 2与 Al(CH 3) 3得到Zn(CH 3 ) 2。因为Al(CH 3 ) 3也可能具有将ZnS转化为Al 2 S 3的能力,所以ZnS ALE的第二种可能的机制是通过TMA的配体交换/转化以及通过HF的氟化。为了验证转化机制,原位四重质谱 (QMS) 研究表明,初始 ZnS 基底上的Al(CH 3 ) 3暴露释放了 Zn(CH 3 ) 2产物,正如转化反应所预期的那样。另外,没有H 2在初始 ZnS 基底上的 HF 暴露过程中,通过 QMS 分析观察到 S 产物。然而,在Al(CH 3 ) 3暴露于ZnS之后,QMS测量监测了HF暴露中的H 2 S,正如预期的,如果Al(CH 3 ) 3将ZnS转化为Al 2 S 3。这些 QMS 结果为 ZnS ALE 期间 ZnS 转化为 Al 2 S 3提供了直接证据。时间依赖性 QMS 结果还表明,转化/配体交换和氟化反应是自限性的。另外,QMS分析观察到Al x F y (CH 3 ) z在 Al(CH 3 ) 3暴露期间作为配体交换产物的二聚体和三聚体。因为Al 2 S 3转化层厚度取决于Al(CH 3 ) 3暴露,所以在同等时间内较大的Al(CH 3 ) 3压力导致较高的ZnS蚀刻速率。相反,在同等时间内较大的 HF 压力对 ZnS 蚀刻速率影响较小,因为 HF 只能氟化转化的 Al 2 S 3层厚度。ZnS 蚀刻速率稍微依赖于 ZnS ALD 生长温度。对于在 100、200 和 300 °C 生长的 ZnS ALD 薄膜,300 °C 下的 ZnS 蚀刻速率分别为 1.4、1.0 和 0.8 Å/周期。在较高温度下生长的 ZnS ALD 薄膜的较低 ZnS 蚀刻速率归因于在较高温度下生长的 ZnS ALD 薄膜具有较大的密度和较高的硫含量。具有较大密度的ZnS ALD膜在Al(CH 3 ) 3反应期间可能更难以从ZnS转化为Al 2 S 3。经过 15 次 ALE 循环后,ZnS 薄膜的 RMS 粗糙度从 7.4 埃略微下降至 5.9 埃。然而,在 30 个 ALE 循环后,RMS 粗糙度随着 ALE 循环逐渐增加。

































 京公网安备 11010802027423号
京公网安备 11010802027423号