当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of (−)-Batrachotoxin Enabled by a Pd/Ag-Promoted Suzuki–Miyaura Coupling Reaction
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-08-15 , DOI: 10.1002/anie.202309688 Yuuki Watanabe 1 , Hisahiro Morozumi 1 , Hiroyuki Mutoh 1 , Koichi Hagiwara 1 , Masayuki Inoue 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-08-15 , DOI: 10.1002/anie.202309688 Yuuki Watanabe 1 , Hisahiro Morozumi 1 , Hiroyuki Mutoh 1 , Koichi Hagiwara 1 , Masayuki Inoue 1
Affiliation
|
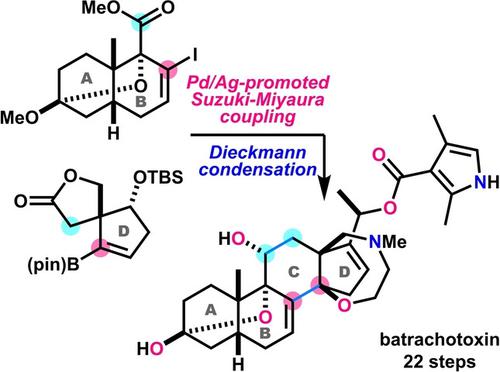
Batrachotoxin is an extremely potent cardio- and neurotoxic steroidal alkaloid. Its 6/6/6/5-membered carbocyclic framework is functionalized with two double bonds, a six-membered hemiacetal, a seven-membered oxazepane, and a dimethylpyrrolecarboxy group. By a novel and simple convergent strategy involving Pd/Ag-promoted Suzuki–Miyaura coupling and Dieckmann condensation reactions, the total synthesis of batrachotoxin was realized in 22 steps.
中文翻译:

Pd/Ag 促进的 Suzuki-Miyaura 偶联反应实现 (−)-Batrachotoxin 的全合成
蝙蝠毒素是一种极强的心脏和神经毒性甾体生物碱。其 6/6/6/5 元碳环骨架由两个双键、一个六元半缩醛、一个七元氧杂氮杂环己烷和一个二甲基吡咯羧基官能化。通过一种新颖而简单的收敛策略,包括 Pd/Ag 促进的 Suzuki-Miyaura 偶联和 Dieckmann 缩合反应,只需 22 个步骤即可实现箭毒毒素的全合成。
更新日期:2023-08-15
中文翻译:

Pd/Ag 促进的 Suzuki-Miyaura 偶联反应实现 (−)-Batrachotoxin 的全合成
蝙蝠毒素是一种极强的心脏和神经毒性甾体生物碱。其 6/6/6/5 元碳环骨架由两个双键、一个六元半缩醛、一个七元氧杂氮杂环己烷和一个二甲基吡咯羧基官能化。通过一种新颖而简单的收敛策略,包括 Pd/Ag 促进的 Suzuki-Miyaura 偶联和 Dieckmann 缩合反应,只需 22 个步骤即可实现箭毒毒素的全合成。































 京公网安备 11010802027423号
京公网安备 11010802027423号