Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2023-08-14 , DOI: 10.1016/j.bioorg.2023.106788 Xiao-Jian Zhang 1 , Fei Yang 1 , Kai-Li Chen 1 , Wei-Mei Fang 1 , Zhi-Qiang Liu 1 , Yu-Guo Zheng 1
|
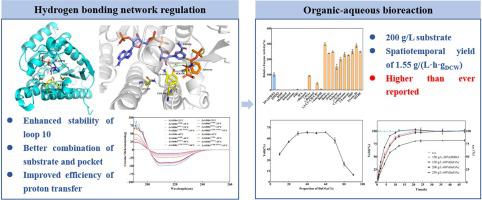
Vibegron is a novel, potent, highly selective β3-adrenergic receptor agonist for the treatment of overactive bladder with higher therapeutic capacity and lower side effects. Methyl(2S,3R)-2-((tert-butoxycarbonyl)amino)-3-hydroxy-3-phenylpropanoate ((2S,3R)-aminohydroxy ester) is a key chiral intermediate for the synthesis of Vibegron. A novel carbonyl reductase from Exiguobacterium sp. s126 (EaSDR6) was isolated using data mining technology from GenBank database with preferable catalytic activity. Hydrogen bond network regulation was performed using site-directed saturation mutagenesis and combination mutagenesis. The mutant EaSDR6A138L/S193A was obtained with the activity improvement by 4.58 folds compared with the wild type EaSDR6. The Km of EaSDR6A138L/S193A was decreased from 1.57 mM to 0.67 mM, kcat was increased by 2.17 folds, and the overall catalytic efficiency kcat/Km was increased by 5.07 folds. The organic-aqueous biphasic bioreaction system for the asymmetric synthesis of (2S,3R)-aminohydroxy ester was constructed for the first time. Under the substrate concentration of 150 g/L, the yield of (2S,3R)-aminohydroxy ester was > 99.99%, the e.e. was > 99.99%, and the spatiotemporal yield was 1.55 g/(L·h·g DCW) after 12 h reaction. While the substrate concentration was increased to 200 g/L and the reaction lasted for 36 h, the yield of (2S,3R)-aminohydroxy ester was > 99.99%, the e.e. was > 99.99% and the spatiotemporal yield was 1.05 g/(L·h·g DCW). The substrate concentration and spatiotemporal yield were higher than ever reported.
中文翻译:

使用基于氢键网络调节分子修饰的新型羰基还原酶高效生物合成 Vibegron 中间体
Vibegron是一种新型、强效、高选择性的β3-肾上腺素能受体激动剂,用于治疗膀胱过度活动症,具有更高的治疗能力和更低的副作用。 ( 2S , 3R )-2-((叔丁氧基羰基)氨基)-3-羟基-3-苯基丙酸甲酯(( 2S , 3R )-氨基羟基酯)是合成Vibegron的关键手性中间体。一种来自微小杆菌属的新型羰基还原酶。利用数据挖掘技术从GenBank数据库中分离得到s126( Ea SDR6),具有较好的催化活性。使用定点饱和诱变和组合诱变进行氢键网络调节。获得突变体Ea SDR6 A138L/S193A,与野生型Ea SDR6相比,活性提高了4.58倍。 Ea SDR6 A138L/S193A的K m从1.57 mM降低至0.67 mM, k cat增加2.17倍,总催化效率k cat / K m增加5.07倍。首次构建了不对称合成( 2S , 3R )-氨基羟基酯的有机-水双相生物反应体系。底物浓度150 g/L下,( 2S , 3R )-氨基羟基酯收率>99.99%,ee>99.99%,时空收率为1.55 g/(L·h·g DCW) )反应12小时后。 当底物浓度增加至200 g/L且反应持续36 h时,(2 S ,3 R )-氨基羟基酯的收率> 99.99%,ee > 99.99%,时空产量为1.05 g /(L·h·g DCW )。底物浓度和时空产率比以往报道的要高。































 京公网安备 11010802027423号
京公网安备 11010802027423号