European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2023-08-14 , DOI: 10.1016/j.ejps.2023.106565 Yun Kuang 1 , Qin Ding 2 , Jie Huang 1 , Shuang Yang 1 , An Yao 1 , Xiaoyan Yang 1 , Min Xiao 3 , Qi Pei 2 , Guoping Yang 4
|
Purpose
This study aimed to assess the pharmacokinetics, safety, and efficacy of GM1 in healthy Chinese subjects and patients with multiple myeloma.
Methods
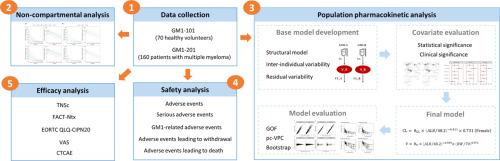
The data used in this study was derived from two dose-escalation trials: GM1-101, involving 70 healthy subjects, and GM1-201, which included 160 multiple myeloma patients. Population pharmacokinetics (PopPK) analysis was conducted on a subset of 90 participants using a nonlinear mixed-effects approach, and potential covariates were explored quantitatively. Observations of any abnormalities in vital signs, physical examinations, laboratory tests, and electrocardiograms during the study period, along with any spontaneously reported and directly observed adverse events, were documented for safety evaluation. Furthermore, neurotoxicity scales were used to assess the efficacy of GM1 as a prophylaxis for chemotherapy-induced peripheral neuropathy and to perform exposure-response analyses in conjunction with pharmacokinetic parameters.
Results
A one-compartment model with first-order elimination best characterized the pharmacokinetics of GM1. The clearance and volume of distribution, as estimated by the final model, were 0.0942 L/h and 3.27 L for GM1-A, and 0.0714 L/h and 2.82 L for GM1-B, respectively. Covariates such as sex, body weight, and albumin significantly influenced pharmacokinetic parameters, yet the variation in steady-state exposure between subjects and reference subjects was less than 45% within their 90% confidence interval. Adverse reactions related to GM1 occurred in 20 (28.6%) and 57 (35.6%) subjects in the GM1-101 and GM1-201 cohorts, respectively. The changes in TNSc and FACT-Ntx scores from baseline at the end of periods 4 and 6 were lower in each GM1 dose group compared to the blank control group. The 400 mg dose group of GM1 displayed greater effectiveness than other dose groups. However, exposure-response analysis revealed no significant modification in efficacy with increasing GM1 exposure.
Conclusions
This study provides the first population pharmacokinetic analysis of GM1. GM1 exhibits a favorable safety profile among healthy subjects and patients with multiple myeloma. GM1 proved effective in mitigating chemotherapy-induced peripheral neuropathy, but this study observed no significant correlation between its efficacy and exposure.
Trial registration numbers
ChiCTR2000041283 and ChiCTR2000041283































 京公网安备 11010802027423号
京公网安备 11010802027423号