Synthesis ( IF 2.2 ) Pub Date : 2023-08-14 , DOI: 10.1055/a-2114-7582 Jumreang Tummatorn 1 , Somsak Ruchirawat 2 , Charnsak Thongsornkleeb 3, 4
|
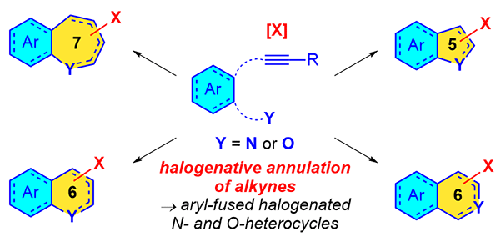
Halogenative annulation of alkyne-tethered N- and O-containing arenes represents a general strategy for the construction of various halogenated N- and O-heterocycles. The methods employed are useful in producing valuable synthetic building blocks carrying C(sp2)–halide functional groups, which are useful synthetic handles, especially for cross-coupling reactions and a myriad of other transformations. When the alkyne is tethered to the heteroatom via an aromatic ring, the reaction gives rise to aryl-fused halogenated heterocycles. In this Short Review, various past and present halogenative annulation methods to construct aryl-fused halogenated N- and O-heterocycles are examined, with a focus on more recent technologies and the various roles of the participating halogenating agents. Additionally, future directions for this age-old, but still very useful, reaction are considered.
1 Introduction
2 Synthesis of Aryl-Fused Halogenated N-Heterocycles
2.1 Aryl-Fused Halogenated 5-Membered N-Heterocycles
2.2 Aryl-Fused Halogenated 6-Membered N-Heterocycles
2.3 Aryl-Fused Halogenated 7-Membered N-Heterocycles
3 Synthesis of Halogenated O-Heterocycles
3.1 Aryl-Fused Halogenated 5-Membered O-Heterocycles
3.2 Aryl-Fused Halogenated 6-Membered O-Heterocycles
3.3 Aryl-Fused Halogenated 7-Membered O-Heterocycles
4 Conclusion
中文翻译:

炔束缚的含 N 和 O 芳烃的卤化环化反应:获取芳基稠合卤代 N 和 O 杂环的方法
炔烃束缚的含N和O芳烃的卤化环化代表了构建各种卤代N和O杂环的一般策略。所采用的方法可用于生产带有 C(sp 2 )–卤化物官能团的有价值的合成结构单元,它们是有用的合成手柄,特别是对于交叉偶联反应和无数其他转化。当炔烃通过芳环连接到杂原子时,反应产生芳基稠合的卤代杂环。在这篇简短的评论中,介绍了过去和现在构建芳基稠合卤代N - 和O的各种卤代环化方法-检查杂环,重点关注最新技术和参与卤化剂的各种作用。此外,还考虑了这种古老但仍然非常有用的反应的未来方向。
1 简介
2 芳基稠合卤代N-杂环化合物的合成
2.1 芳基稠合卤代五元N-杂环
2.2 芳基稠合卤代六元N-杂环
2.3 芳基稠合卤代七元N-杂环
3 卤代O-杂环化合物的合成
3.1 芳基稠合卤代五元O-杂环
3.2 芳基稠合卤代六元O-杂环
3.3 芳基稠合卤代7元O-杂环
4。结论































 京公网安备 11010802027423号
京公网安备 11010802027423号