Nano Research ( IF 9.5 ) Pub Date : 2023-08-14 , DOI: 10.1007/s12274-023-5899-0 Xusheng Cheng , Jinwen Hu , Wenzhe Shang , Jingya Guo , Cuncun Xin , Songlin Zhang , Suchan Song , Wei Liu , Yantao Shi
|
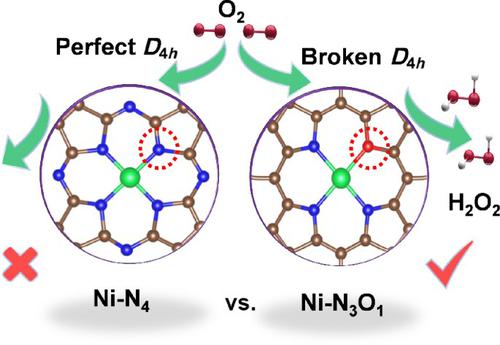
Atomic transition-metal-nitrogen-carbon electrocatalysts hold great promise as alternatives to benchmark Pt in the oxygen reduction reaction. The pristine metal centers with quasi square-planar D4h configuration, however, still suffer from unfavorable energetics and thereby strong activity/selectivity trade-off during the catalytic process. Here we present a ligand-field engineering of single-atom Ni-N-C catalysts to boost the sluggish kinetics via rationally constructing prototypical asymmetrically ligated Ni-N3O1 sites. The as-obtained Ni-supported multi-walled carbon nanotubes with molten salt-treated (defined as Ni/CNS) catalyst delivered an excellent H2O2 selectivity (> 90%) within a wide potential window (0.2–0.7 V vs. reversible hydrogen electrode (RHE)) and robust stability (for 10 h) in alkaline medium. Combined electron paramagnetic resonance and theoretical analysis rationalize this finding and demonstrate that the broken symmetry facilitates the electron transfer of a σ* to O-O orbital as compared to the Ni-N4 counterpart, playing an indispensable role in efficient O2 activation.
中文翻译:

用于高效过氧化氢电合成的不对称连接单原子镍位点
原子过渡金属-氮-碳电催化剂作为氧还原反应中基准铂的替代品具有广阔的前景。然而,具有准方形平面D 4 h构型的原始金属中心仍然遭受不利的能量学影响,从而在催化过程中产生强烈的活性/选择性权衡。在这里,我们提出了单原子 Ni-NC 催化剂的配体场工程,通过合理构建原型不对称连接的 Ni-N 3 O 1位点来提高缓慢的动力学。所获得的镍负载多壁碳纳米管与熔盐处理(定义为 Ni/CNS)催化剂可提供优异的 H 2 O 2在宽电位窗口(0.2–0.7 V 与可逆氢电极 (RHE))内具有选择性(> 90%),并且在碱性介质中具有强大的稳定性(10 小时)。结合电子顺磁共振和理论分析合理化了这一发现,并证明与 Ni-N 4对应物相比,对称性破缺促进了 σ* 到 OO 轨道的电子转移,在有效的 O 2 活化中发挥着不可或缺的作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号