当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photochemical Isomerization of Cyclohept-1-ene-1-carbaldehyde: Strain-Release Cycloadditions and Ene Reactions
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-08-14 , DOI: 10.1021/acs.joc.3c01311 Daniel P Schwinger 1 , Thomas Pickl 1 , Thorsten Bach 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-08-14 , DOI: 10.1021/acs.joc.3c01311 Daniel P Schwinger 1 , Thomas Pickl 1 , Thorsten Bach 1
Affiliation
|
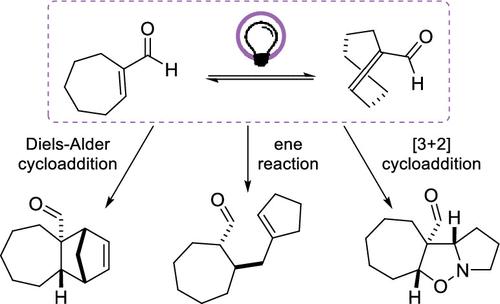
Cyclohept-1-ene-1-carbaldehyde undergoes photoinduced E → Z isomerization at λ = 350 nm. The ring strain facilitates Diels–Alder cycloaddiions with 1,3-dienes, [3 + 2] cycloadditions with 1,3-dipoles, and ene reactions with olefins. Products are trans-fused at the cycloheptane core and were obtained in yields of up to 82%. Single crystal X-ray analyses corroborated the constitution and relative configuration of key products. With BF3 as a Lewis acid and 2,3-dimethylbuta-1,3-diene, cyclohept-1-ene-1-carbaldehyde reacted in the dark and rearranged stereoselectively to a tricyclic ketone (87%).
中文翻译:

环庚-1-烯-1-甲醛的光化学异构化:应变释放环加成和烯反应
环庚-1-烯-1-甲醛在 λ = 350 nm 处发生光诱导E → Z异构化。环应变促进了与 1,3-二烯的狄尔斯-阿尔德环加成反应、与 1,3-偶极子的 [3 + 2] 环加成反应以及烯与烯烃的反应。产物在环庚烷核心处进行转融合,收率高达 82%。单晶X射线分析证实了关键产物的构成和相关构型。以BF 3作为路易斯酸与2,3-二甲基丁-1,3-二烯、环庚-1-烯-1-甲醛在黑暗中反应并立体选择性重排生成三环酮(87%)。
更新日期:2023-08-14
中文翻译:

环庚-1-烯-1-甲醛的光化学异构化:应变释放环加成和烯反应
环庚-1-烯-1-甲醛在 λ = 350 nm 处发生光诱导E → Z异构化。环应变促进了与 1,3-二烯的狄尔斯-阿尔德环加成反应、与 1,3-偶极子的 [3 + 2] 环加成反应以及烯与烯烃的反应。产物在环庚烷核心处进行转融合,收率高达 82%。单晶X射线分析证实了关键产物的构成和相关构型。以BF 3作为路易斯酸与2,3-二甲基丁-1,3-二烯、环庚-1-烯-1-甲醛在黑暗中反应并立体选择性重排生成三环酮(87%)。































 京公网安备 11010802027423号
京公网安备 11010802027423号