Nano Research ( IF 9.5 ) Pub Date : 2023-08-14 , DOI: 10.1007/s12274-023-5978-2 Qiang Yang , Ningyu Liu , Ziyin Zhao , Xun Liu , Lichen Yin
|
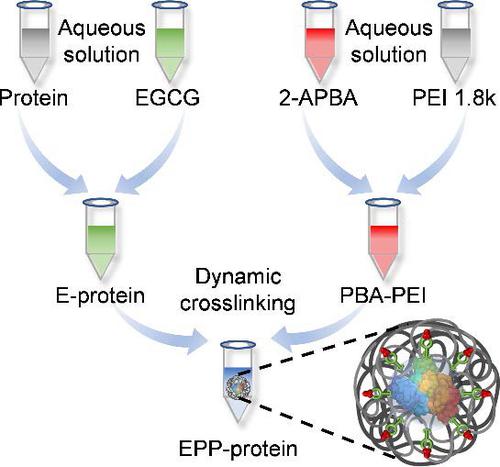
Intracellular protein delivery is critical to the development of protein-based biopharmaceuticals and therapies. However, current delivery vectors often suffer from complicated syntheses, low generality among various proteins, and insufficient serum stability. Herein, we developed an enlightened cytosolic protein delivery strategy by dynamically crosslinking epigallocatechin gallate (EGCG), low-molecular-weight polyethylenimine (PEI 1.8k), and 2-acetylphenylboric acid (2-APBA) on the protein surface, hence forming the EPP-protein nanocapsules (NCs). EGCG enhanced protein encapsulation via hydrogen bonding, and reduced the positive charge density of PEI to endow the NCs with high serum tolerance, thereby enabling effective cellular internalization in serum. The formation of reversible imine and boronate ester among 2-APBA, EGCG, and PEI 1.8k allowed acid-triggered dissociation of EPP-protein NCs in the endolysosomes, which triggered efficient intracellular release of the native proteins. Such strategy therefore showed high efficiency and universality for diversities of proteins with different molecular weights and isoelectric points, including enzyme, toxin, antibody, and CRISPR (clustered regularly interspaced short palindromic repeats)-Cas9 ribonucleoprotein (RNP), outperforming the commercial protein transduction reagent PULSin and RNP transfection reagent lipofectamine CMAX. Moreover, intravenously (i.v.) injected EPP-saporin NCs efficiently delivered saporin into 4T1 tumor cells to provoke robust antitumor effect. This simple, versatile, and robust cytosolic protein delivery system holds translational potentials for the development of protein-based therapeutics.
中文翻译:

动态交联纳米胶囊可实现高效且抗血清的胞质蛋白递送
细胞内蛋白质递送对于基于蛋白质的生物制药和疗法的开发至关重要。然而,目前的递送载体常常存在合成复杂、各种蛋白质之间的通用性低以及血清稳定性不足的问题。在此,我们开发了一种启发式胞质蛋白递送策略,通过在蛋白质表面动态交联表没食子儿茶素没食子酸酯(EGCG)、低分子量聚乙烯亚胺(PEI 1.8k)和2-乙酰苯基硼酸(2-APBA),从而形成EPP -蛋白质纳米胶囊(NC)。EGCG通过氢键增强蛋白质封装,并降低PEI的正电荷密度,赋予NCs高血清耐受性,从而实现血清中有效的细胞内化。2-APBA、EGCG 和 PEI 1 之间形成可逆亚胺和硼酸酯。8k 允许酸触发内溶酶体中 EPP 蛋白 NC 的解离,从而触发天然蛋白的有效细胞内释放。因此,这种策略对不同分子量和等电点的蛋白质多样性表现出高效性和普适性,包括酶、毒素、抗体和CRISPR(成簇规则间隔短回文重复序列)-Cas9核糖核蛋白(RNP),优于商业蛋白质转导试剂PULSin 和 RNP 转染试剂 lipofectamine CMAX。此外,静脉注射(因此,这种策略对不同分子量和等电点的蛋白质多样性表现出高效性和普适性,包括酶、毒素、抗体和CRISPR(成簇规则间隔短回文重复序列)-Cas9核糖核蛋白(RNP),优于商业蛋白质转导试剂PULSin 和 RNP 转染试剂 lipofectamine CMAX。此外,静脉注射(因此,这种策略对不同分子量和等电点的蛋白质多样性表现出高效性和普适性,包括酶、毒素、抗体和CRISPR(成簇规则间隔短回文重复序列)-Cas9核糖核蛋白(RNP),优于商业蛋白质转导试剂PULSin 和 RNP 转染试剂 lipofectamine CMAX。此外,静脉注射(iv ) 注射 EPP-皂草素 NCs 有效地将皂草素递送到 4T1 肿瘤细胞中,从而激发强大的抗肿瘤作用。这种简单、多功能且强大的胞质蛋白递送系统具有开发基于蛋白质的疗法的转化潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号