Molecular Catalysis ( IF 3.9 ) Pub Date : 2023-08-12 , DOI: 10.1016/j.mcat.2023.113440 Bhaskarjyoti Sarma , Rishi Ranjan , Nimesh R. Chauhan , Suman Mukhopadhyay , Angshuman Roy Choudhury , Komal M. Vyas
|
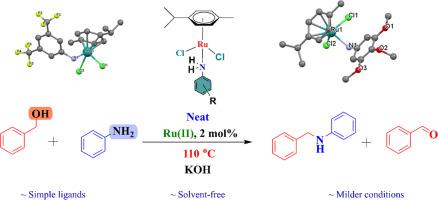
Efficient Ru(II) catalysts with substituted aniline based ligands were synthesized having chemical formula [Ru(η6-p-cymene)Cl2(L1)] [Ru-1] and [Ru(η6-p-cymene)Cl2(L2)] [Ru-2] (where, L1 = 3,5-bis(trifluoromethyl)aniline and L2 = 3,4,5- trimethoxy aniline). The structural features of both the complexes were authenticated by ESI-MS, 1H and 13C NMR, FT-IR, elemental analyses, and single-crystal X-ray diffraction studies. Catalytic performance of both [Ru-1] and [Ru-2] was investigated for conversion of primary amine to secondary amine using alcohols under milder and solvent-free conditions and compared with the reported aniline-based Ru(II)-arene complex [Ru-3]. Among them, [Ru-1] could achieve 100% selectivity towards mono N-alkylated amines with > 99% conversion without formation of any imine derivatives. The results suggested that the lone pair of electrons of benzyl alcohol binds easily to the electron-deficient Ru(II) center of [Ru-1] catalyst making it more effective compared to electron-rich [Ru-2] and [Ru-3] catalysts. The catalytic performance of [Ru-1] demonstrates that it is one of the most active primary amine ligated Ru(II) based catalytic systems for N-alkylation of anilines under milder and solvent-free conditions.
中文翻译:

高活性伯胺连接的 Ru(II)-芳烃配合物作为苯胺无溶剂 N-烷基化的选择性催化剂
合成了具有取代苯胺基配体的高效Ru(II)催化剂,其化学式为[Ru(η 6 -对伞花烃)Cl 2 (L1)] [Ru-1]和[Ru(η 6 -对伞花烃)Cl 2 (L2)] [Ru-2](其中,L1 = 3,5-双(三氟甲基)苯胺且 L2 = 3,4,5-三甲氧基苯胺)。两种配合物的结构特征均通过 ESI-MS、1 H 和13 C NMR、FT-IR、元素分析和单晶 X 射线衍射研究进行了验证。[Ru-1]和[Ru-2]的催化性能研究了在较温和且无溶剂的条件下使用醇将伯胺转化为仲胺的情况,并与报道的苯胺基 Ru(II)-芳烃络合物[Ru-3]进行比较。其中,[Ru-1]对单N-烷基化胺具有100%的选择性,转化率>99%,且不形成任何亚胺衍生物。结果表明,苯甲醇的孤对电子很容易与[Ru-1]催化剂的缺电子Ru(II)中心结合,使其比富电子的[Ru-2]和[Ru-3]更有效]催化剂。[Ru-1]的催化性能证明它是最活跃的伯胺连接 Ru(II) 基催化体系之一,用于在温和和无溶剂条件下进行苯胺 N-烷基化。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号