Minerals Engineering ( IF 4.9 ) Pub Date : 2023-08-10 , DOI: 10.1016/j.mineng.2023.108308 Yasemin Ozturk
|
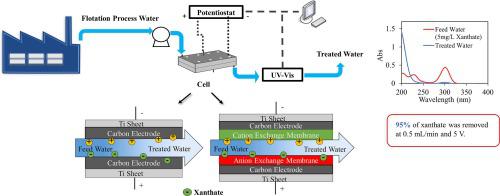
In this work, the removal of xanthate from flotation process water by electrochemical advanced oxidation processes (EAOPs) was investigated. An electrochemical cell was fabricated using a pair of carbon electrodes as the anode and cathode. Electrodes were prepared by depositing an aqueous slurry of activated carbon and PVA binder onto graphite sheets. Carbon disulfide (CS2) was produced during the process and removed to some extent by absorption of carbon electrodes and/or further oxidation to sulfate (SO42−).
The effect of operating parameters such as applied voltage, flow rate, and influent concentration on process efficiency was evaluated to achieve efficient xanthate removal. Application of various potentials ranging from 1 V to 7 V showed that xanthate removal efficiency increased at higher voltages. The effluent concentration was cleaner and the salt removal efficiency was higher at slower flow rates. The highest removal efficiency of 95% was achieved at a flow rate of 0.5 mL/min. Since the density of ions between the electrodes increased with increasing influent concentration, the total xanthate removal increased from 599.4 mg/m2 at 5 mg/L influent concentration to 12834.2 mg/m2 at 40 mg/L. Long-term stability tests showed that electrode performance degraded steadily and the removal efficiency decreased from 85% to 56% in 30 h.
Batch mode experiments in the presence of ion exchange membranes showed that xanthate was oxidized to CS2 at 0.8 V and 1.0 V and to monothiocarbonate (ROCSO−) at 1.2 V. 96% of xanthate was removed from 5 mg/L xanthate solution at a flow rate of 0.5 mL/min at 1.0 V.
The effect of real mine water components on xanthate removal efficiency was investigated by using flotation process water from a copper mine in Turkey. The results showed that the process efficiency decreased from 67% to 32% in the presence of mine water, which could be attributed to electrode fouling.
中文翻译:

电化学高级氧化去除浮选工艺水中的黄药
在这项工作中,研究了通过电化学高级氧化工艺(EAOP)从浮选工艺水中去除黄药。使用一对碳电极作为阳极和阴极制造电化学电池。通过将活性炭和 PVA 粘合剂的水浆沉积到石墨片上来制备电极。在此过程中产生二硫化碳(CS 2 ),并通过碳电极的吸收和/或进一步氧化成硫酸盐(SO 4 2− )在一定程度上去除。
评估了施加电压、流速和进水浓度等操作参数对工艺效率的影响,以实现有效的黄药去除。应用 1 V 至 7 V 的各种电位表明,黄药去除效率在较高电压下增加。流速较慢时,出水浓度更清洁,除盐效率更高。当流速为 0.5 mL/min 时,去除效率最高可达 95%。由于电极之间的离子密度随着进水浓度的增加而增加,总黄药去除率从5 mg/ L进水浓度时的599.4 mg/m 2增加到12834.2 mg/m 240毫克/升。长期稳定性测试表明,电极性能稳步下降,去除效率在30小时内从85%下降至56%。
在离子交换膜存在下的批量模式实验表明,黄原酸盐在 0.8 V 和 1.0 V 下被氧化为 CS 2,在 1.2 V 下被氧化为一硫代碳酸盐 (ROCSO - )。在 5 mg/L 黄原酸盐溶液中,96% 的黄原酸盐在1.0 V 时流速为 0.5 mL/min。
使用土耳其铜矿的浮选工艺水,研究了真实矿井水成分对黄药去除效率的影响。结果表明,在存在矿井水的情况下,工艺效率从 67% 下降到 32%,这可能是由于电极结垢造成的。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号