Fuel ( IF 6.7 ) Pub Date : 2023-08-11 , DOI: 10.1016/j.fuel.2023.129402
Ye Jiang , Yinsheng Jiang , Congcong Su , Xin Sun , Yichao Xu , Siyuan Cheng , Yanan Liu , Xiao Dou , Zhengda Yang
|
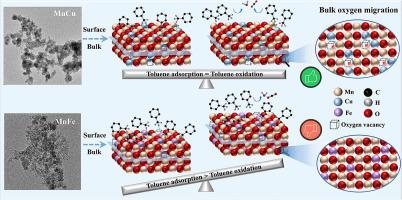
α-MnO2 doped with four metals (Cu, Ce, Co, Fe) were prepared via a redox co-precipitation method. MnCu exhibited the highest activity and the temperature required for achieving 90% toluene conversion was 224℃ at a weight hourly space velocity of 30000 mL·g−1·h−1. Moreover, MnCu possessed outstanding thermal stability and resistance to H2O. The optimal crystal structure, appropriate Mn3+/Mn4+ proportion, and superior redox properties of MnCu contributed to the generation of oxygen vacancies and the highest lattice oxygen (Olat) mobility. Both surface and bulk lattice oxygen consumed in MnCu can be supplemented promptly by gaseous oxygen. Therefore, a balance between toluene adsorption and deep oxidation was built in MnCu to ensure the continuous oxidation of toluene. Although the abundant surface defects and a relatively active surface lattice oxygen accelerated the adsorption and activation of toluene over MnFe, it exhibited the poorest activity. This was because the deep oxidation of toluene was inhibited owing to poor lattice oxygen migration. The adsorbed toluene and part intermediates might cover the catalyst surface preventing the continuous oxidation of toluene. This further illustrated that the deep oxidation of toluene was much more important than the adsorption for the design of MnO2-based mixed oxide catalysts.
中文翻译:

阐明不同金属掺杂对α-MnO2对甲苯吸附和深度氧化的影响
采用氧化还原共沉淀法制备了Cu、Ce、Co、Fe四种金属掺杂的α-MnO 2 。MnCu表现出最高的活性,在重时空速30000 mL·g -1 ·h -1下实现90%甲苯转化率所需的温度为224℃ 。此外,MnCu具有优异的热稳定性和抗H 2 O能力。MnCu的最佳晶体结构、适当的Mn 3+ /Mn 4+比例以及优异的氧化还原性能有助于氧空位的产生和最高的晶格氧(O lat)流动性。MnCu 中消耗的表面和体晶格氧都可以通过气态氧及时补充。因此,MnCu中建立了甲苯吸附和深度氧化之间的平衡,以保证甲苯的连续氧化。尽管丰富的表面缺陷和相对活跃的表面晶格氧加速了MnFe对甲苯的吸附和活化,但其活性最差。这是因为晶格氧迁移不良,抑制了甲苯的深度氧化。吸附的甲苯和部分中间体可能覆盖催化剂表面,阻止甲苯的连续氧化。这进一步说明对于MnO 2基混合氧化物催化剂的设计来说,甲苯的深度氧化比吸附更为重要。

































 京公网安备 11010802027423号
京公网安备 11010802027423号