当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Radical Cyclization Cascades Triggered by Addition of Diverse Radicals to Alkynes To Construct 6(5)–6–5 Fused Rings
Organic Letters ( IF 4.9 ) Pub Date : 2016-10-04 00:00:00 , DOI: 10.1021/acs.orglett.6b02599 Lin Huang 1 , Liu Ye 1 , Xiao-Hua Li 1 , Zhong-Liang Li 1 , Jin-Shun Lin 1 , Xin-Yuan Liu 1
Organic Letters ( IF 4.9 ) Pub Date : 2016-10-04 00:00:00 , DOI: 10.1021/acs.orglett.6b02599 Lin Huang 1 , Liu Ye 1 , Xiao-Hua Li 1 , Zhong-Liang Li 1 , Jin-Shun Lin 1 , Xin-Yuan Liu 1
Affiliation
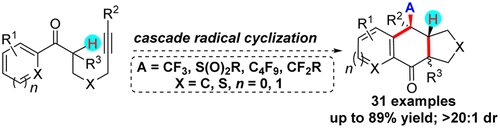
|
Cascade radical cyclization of alkynyl ketones with various carbon- and heteroatom-centered radical precursors via a sequential radical addition/1,5-H radical shift/5-exo-trig/radical cyclization process was realized for the first time. This method provides a strategically novel and step-economical protocol for diversity-oriented synthesis of a wide range of carbocyclic and heterocyclic 6(5)–6–5 fused ring systems with three contiguous stereocenters, including a quaternary carbon in high yields with excellent chemo- and diastereoselectivity.
中文翻译:

通过向炔烃中添加多种自由基触发立体选择性自由基环化级联,以构建6(5)–6-5稠环
首次实现了通过连续的自由基加成/ 1,5-H自由基转移/ 5 -exo-trig /自由基环化过程,将具有各种以碳原子和杂原子为中心的自由基前体进行的炔基酮的级联自由基环化。该方法为广泛的具有三个连续立体中心的碳环和杂环6(5)–6–5稠合环系统的多样性导向合成提供了战略上新颖且经济的步骤,其中包括高产率的季碳和出色的化学-和非对映选择性。
更新日期:2016-10-04
中文翻译:

通过向炔烃中添加多种自由基触发立体选择性自由基环化级联,以构建6(5)–6-5稠环
首次实现了通过连续的自由基加成/ 1,5-H自由基转移/ 5 -exo-trig /自由基环化过程,将具有各种以碳原子和杂原子为中心的自由基前体进行的炔基酮的级联自由基环化。该方法为广泛的具有三个连续立体中心的碳环和杂环6(5)–6–5稠合环系统的多样性导向合成提供了战略上新颖且经济的步骤,其中包括高产率的季碳和出色的化学-和非对映选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号