当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Converging PMF Calculations of Antibiotic Permeation across an Outer Membrane Porin with Subkilocalorie per Mole Accuracy
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2023-08-10 , DOI: 10.1021/acs.jcim.3c00880 Jeremy Lapierre 1 , Jochen S Hub 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2023-08-10 , DOI: 10.1021/acs.jcim.3c00880 Jeremy Lapierre 1 , Jochen S Hub 1
Affiliation
|
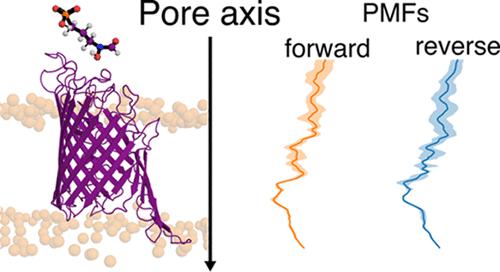
The emergence of multidrug-resistant pathogens led to a critical need for new antibiotics. A key property of effective antibiotics against Gram-negative bacteria is their ability to permeate through the bacterial outer membrane via transmembrane porin proteins. Molecular dynamics (MD) simulations are, in principle, capable of modeling antibiotic permeation across outer membrane porins (OMPs). However, owing to sampling problems, it has remained challenging to obtain converged potentials of mean force (PMFs) for antibiotic permeation across OMPs. Here, we investigated the convergence of PMFs along a single collective variable aimed at quantifying the permeation of the antibiotic fosmidomycin across the OprO porin. We compared standard umbrella sampling (US) with three advanced flavors of the US technique: (i) Hamiltonian replica exchange with solute tempering in combination with US, (ii) simulated tempering-enhanced US, and (iii) replica-exchange US. To quantify the PMF convergence and to reveal hysteresis problems, we computed several independent sets of US simulations starting from pulling simulations in the outward and inward permeation directions. We find that replica-exchange US in combination with well-chosen restraints is highly successful for obtaining converged PMFs of fosmidomycin permeation through OprO, reaching PMFs converged to subkilocalorie per mole accuracy.
中文翻译:

抗生素跨外膜孔蛋白渗透的 PMF 计算收敛,每摩尔亚千卡精度
多重耐药病原体的出现导致对新型抗生素的迫切需求。有效对抗革兰氏阴性细菌的抗生素的一个关键特性是它们能够通过跨膜孔蛋白渗透细菌外膜。原则上,分子动力学 (MD) 模拟能够模拟抗生素跨外膜孔蛋白 (OMP) 的渗透。然而,由于采样问题,获得抗生素跨 OMP 渗透的平均力 (PMF) 的收敛势仍然具有挑战性。在这里,我们研究了 PMF 沿着单个集体变量的收敛性,旨在量化抗生素 Fosmidomycin 穿过 OprO 孔蛋白的渗透。我们将标准伞式采样 (US) 与三种先进的 US 技术进行了比较:(i) 哈密顿复制品交换与溶质回火结合 US,(ii) 模拟回火增强 US,以及 (iii) 复制品交换 US。为了量化 PMF 收敛并揭示滞后问题,我们从向外和向内渗透方向的拉动模拟开始,计算了几组独立的 US 模拟。我们发现,复制品交换 US 与精心选择的约束相结合,可以非常成功地获得通过 OprO 的膦米霉素渗透的收敛 PMF,达到收敛到每摩尔亚千卡精度的 PMF。
更新日期:2023-08-10
中文翻译:

抗生素跨外膜孔蛋白渗透的 PMF 计算收敛,每摩尔亚千卡精度
多重耐药病原体的出现导致对新型抗生素的迫切需求。有效对抗革兰氏阴性细菌的抗生素的一个关键特性是它们能够通过跨膜孔蛋白渗透细菌外膜。原则上,分子动力学 (MD) 模拟能够模拟抗生素跨外膜孔蛋白 (OMP) 的渗透。然而,由于采样问题,获得抗生素跨 OMP 渗透的平均力 (PMF) 的收敛势仍然具有挑战性。在这里,我们研究了 PMF 沿着单个集体变量的收敛性,旨在量化抗生素 Fosmidomycin 穿过 OprO 孔蛋白的渗透。我们将标准伞式采样 (US) 与三种先进的 US 技术进行了比较:(i) 哈密顿复制品交换与溶质回火结合 US,(ii) 模拟回火增强 US,以及 (iii) 复制品交换 US。为了量化 PMF 收敛并揭示滞后问题,我们从向外和向内渗透方向的拉动模拟开始,计算了几组独立的 US 模拟。我们发现,复制品交换 US 与精心选择的约束相结合,可以非常成功地获得通过 OprO 的膦米霉素渗透的收敛 PMF,达到收敛到每摩尔亚千卡精度的 PMF。































 京公网安备 11010802027423号
京公网安备 11010802027423号