当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microwave-Assisted Palladium Acetate-Catalyzed C–P Cross-Coupling of Arylboronic Acids and >P(O)H Reagents in the Absence of the Usual Mono- and Bidentate P-Ligands: Mechanistic Insights
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-08-09 , DOI: 10.1021/acs.joc.3c01269
Bianka Huszár 1 , Zoltán Mucsi 1, 2 , György Keglevich 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-08-09 , DOI: 10.1021/acs.joc.3c01269
Bianka Huszár 1 , Zoltán Mucsi 1, 2 , György Keglevich 1
Affiliation
|
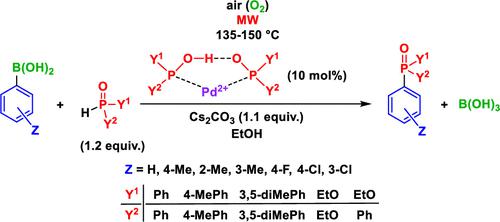
A less-studied halogen-free variation of the Hirao reaction involving the coupling of arylboronic acids with >P(O)H reagents, such as diarylphosphine oxides, diethyl phosphite, and ethyl phenyl-H-phosphinate, was investigated in detail using Pd(OAc)2 as the catalyst precursor and applying some excess of the P-reagent to supply the ligand via its trivalent tautomeric (>P–OH) form. The optimum conditions (1.2 equiv of the P-reagent, 135–150 °C, and air) were explored for the synthesis of diaryl-phenylphosphine oxides, aryl-diphenylphosphine oxides, diethyl arylphosphonates, ethyl diphenylphosphinate, and two bisphosphinoyl derivatives. In the reaction of 4-chlorophenyl- or 3-chlorophenylboronic acid with Ph2P(O)H, triphenylphosphine oxide was also formed as a byproduct. Theoretical calculations suggested that the catalytic cycle of the P–C coupling of PhB(OH)2 with Ph2P(O)H is different from that of the usual cross-coupling reactions. It comprises the addition of a phenyl anion and then the tautomeric form >P–OH of the >P(O)H reagent to the Pd2+ catalyst complex. This is then followed by reductive elimination affording Ph3PO that is accompanied with the conversion of Pd2+ to Pd0. There is a need for a subsequent stoichiometric oxidation of Pd(0) by molecular oxygen. The spontaneous formation of the self-assembling ligands around the Pd2+ center from the >P(O)H reactant plays a crucial role in the mechanism and promotes the efficiency of the catalyst.
中文翻译:

在没有常用单齿和双齿 P-配体的情况下,微波辅助乙酸钯催化芳基硼酸和 >P(O)H 试剂的 C-P 交叉偶联:机理见解
使用Pd ( OAc) 2作为催化剂前体,并应用一些过量的 P 试剂通过其三价互变异构 (>P-OH) 形式提供配体。探索了合成二芳基苯基氧化膦、芳基二苯基氧化膦、芳基膦酸二乙酯、二苯基次膦酸乙酯和两种双膦酰基衍生物的最佳条件(1.2当量的P试剂、135-150°C和空气)。在4-氯苯基-或3-氯苯基硼酸与Ph 2 P(O)H的反应中,还形成副产物三苯基氧化膦。理论计算表明PhB(OH) 2与Ph 2 P(O)H的P-C偶联催化循环与通常的交叉偶联反应不同。它包括添加苯基阴离子,然后将 >P(O)H 试剂的互变异构形式 >P-OH 添加到 Pd 2+催化剂络合物中。然后进行还原消除,得到Ph 3 PO,同时将Pd 2+转化为Pd 0。需要通过分子氧对 Pd(0) 进行后续化学计量氧化。>P(O)H 反应物在Pd 2+中心周围自发形成的自组装配体在该机理中起着至关重要的作用,并提高了催化剂的效率。
更新日期:2023-08-10
中文翻译:

在没有常用单齿和双齿 P-配体的情况下,微波辅助乙酸钯催化芳基硼酸和 >P(O)H 试剂的 C-P 交叉偶联:机理见解
使用Pd ( OAc) 2作为催化剂前体,并应用一些过量的 P 试剂通过其三价互变异构 (>P-OH) 形式提供配体。探索了合成二芳基苯基氧化膦、芳基二苯基氧化膦、芳基膦酸二乙酯、二苯基次膦酸乙酯和两种双膦酰基衍生物的最佳条件(1.2当量的P试剂、135-150°C和空气)。在4-氯苯基-或3-氯苯基硼酸与Ph 2 P(O)H的反应中,还形成副产物三苯基氧化膦。理论计算表明PhB(OH) 2与Ph 2 P(O)H的P-C偶联催化循环与通常的交叉偶联反应不同。它包括添加苯基阴离子,然后将 >P(O)H 试剂的互变异构形式 >P-OH 添加到 Pd 2+催化剂络合物中。然后进行还原消除,得到Ph 3 PO,同时将Pd 2+转化为Pd 0。需要通过分子氧对 Pd(0) 进行后续化学计量氧化。>P(O)H 反应物在Pd 2+中心周围自发形成的自组装配体在该机理中起着至关重要的作用,并提高了催化剂的效率。

































 京公网安备 11010802027423号
京公网安备 11010802027423号