Separation and Purification Technology ( IF 8.1 ) Pub Date : 2023-08-09 , DOI: 10.1016/j.seppur.2023.124763
Dunyu Sun , Bingyu Shen , Shaogui Yang , Xinying Cheng , Qiang Zhong , Syed Azhar Abbas , Yinhao Dai , Yazi Liu , Chenmin Xu , Chengdu Qi , Huan He , Shiyin Li
|
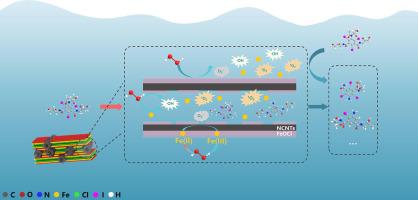
Heterogeneous Fenton has already been used extensively to remove emerging contaminants, however, sluggish Fe(III)/Fe(II) circulation and high mass-transport resistance are its two major limitations. In this study, a novel heterogeneous N-doped carbon nanotubes loaded nano-FeOCl (NCF) catalyst was synthesized by partial thermal decomposition to enhance its catalytic performance. By incorporating NCNTs, the specific surface area of NCF was significantly increased in comparison to FeOCl, which promotes the accumulation of contaminants on the catalyst surface. Similarly, the degradation rate constant (k) of IOH in the NCF/H2O2 system was observed 114 times greater than that of the FeOCl/H2O2 system. Besides not significantly increased k, excessive H2O2 substantially reduced H2O2 utilization. Electron paramagnetic resonance and electrochemical analysis reflected that the NCNTs facilitated the Fe(III)/Fe(II) circulation by improving electron transfer and enhanced •OH, O2•− and 1O2 availability by exerting a confinement effect. The major reactions assumed in IOH degradation include hydrogen atom abstract, amide hydrolysis, deiodination reaction, •OH radical adduct formation, and oxidation of C-OH. This study elucidates the degradation mechanism of IOH in the heterogeneous Fenton system with carbon-based co-catalysts and provides an efficient measure for removing emerging contaminants from the environment.
中文翻译:

氮掺杂碳纳米管增强 FeOCl 去除 IOH 的非均相芬顿反应:NCNT 的作用和机制
非均相芬顿已被 广泛用于去除新出现的污染物,然而,Fe(III)/Fe(II)循环缓慢和传质阻力大是其两个主要限制。在这项研究中,通过部分热分解合成了一种新型异质氮掺杂碳纳米管负载纳米FeOCl(NCF)催化剂,以增强其催化性能。通过掺入 NCNT,NCF 的比表面积 与 FeOCl 相比显着增加,这促进了污染物在催化剂表面的积累。类似地, NCF/H 2 O 2体系中 IOH 的降解速率常数(k )比 FeOCl/H 2 O体系中的降解速率常数大 114 倍。2系统。除了没有显着增加k之外,过量的H 2 O 2显着降低了H 2 O 2的利用率。电子顺磁共振和电化学分析表明,NCNT通过改善电子传递和增强的•OH、O 2 •−和1 O 2促进了Fe(III)/Fe(II)循环。通过发挥限制效应来实现可用性。IOH降解中假定的主要反应包括氢原子抽象、酰胺水解、脱碘反应、•OH自由基加合物形成和C-OH氧化。本研究阐明了含碳助催化剂的非均相芬顿体系中 IOH 的降解机制,并为去除环境中新出现的污染物提供了有效的措施。

































 京公网安备 11010802027423号
京公网安备 11010802027423号