Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Humanized mouse liver reveals endothelial control of essential hepatic metabolic functions
Cell ( IF 45.5 ) Pub Date : 2023-08-09 , DOI: 10.1016/j.cell.2023.07.017 Eleanna Kaffe 1 , Manolis Roulis 1 , Jun Zhao 2 , Rihao Qu 2 , Esen Sefik 1 , Haris Mirza 3 , Jing Zhou 1 , Yunjiang Zheng 1 , Georgia Charkoftaki 4 , Vasilis Vasiliou 4 , Daniel F Vatner 5 , Wajahat Z Mehal 6 , 7 , Yuval Kluger 8 , Richard A Flavell 9
Cell ( IF 45.5 ) Pub Date : 2023-08-09 , DOI: 10.1016/j.cell.2023.07.017 Eleanna Kaffe 1 , Manolis Roulis 1 , Jun Zhao 2 , Rihao Qu 2 , Esen Sefik 1 , Haris Mirza 3 , Jing Zhou 1 , Yunjiang Zheng 1 , Georgia Charkoftaki 4 , Vasilis Vasiliou 4 , Daniel F Vatner 5 , Wajahat Z Mehal 6 , 7 , Yuval Kluger 8 , Richard A Flavell 9
Affiliation
|
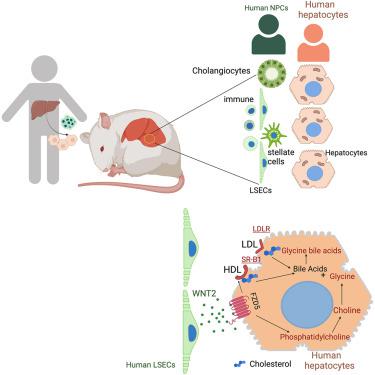
Hepatocytes, the major metabolic hub of the body, execute functions that are human-specific, altered in human disease, and currently thought to be regulated through endocrine and cell-autonomous mechanisms. Here, we show that key metabolic functions of human hepatocytes are controlled by non-parenchymal cells (NPCs) in their microenvironment. We developed mice bearing human hepatic tissue composed of human hepatocytes and NPCs, including human immune, endothelial, and stellate cells. Humanized livers reproduce human liver architecture, perform vital human-specific metabolic/homeostatic processes, and model human pathologies, including fibrosis and non-alcoholic fatty liver disease (NAFLD). Leveraging species mismatch and lipidomics, we demonstrate that human NPCs control metabolic functions of human hepatocytes in a paracrine manner. Mechanistically, we uncover a species-specific interaction whereby WNT2 secreted by sinusoidal endothelial cells controls cholesterol uptake and bile acid conjugation in hepatocytes through receptor FZD5. These results reveal the essential microenvironmental regulation of hepatic metabolism and its human-specific aspects.
中文翻译:

人源化小鼠肝脏揭示内皮细胞对肝脏基本代谢功能的控制
肝细胞是人体的主要代谢中心,执行人类特有的功能,在人类疾病中改变,目前认为通过内分泌和细胞自主机制进行调节。在这里,我们表明人类肝细胞的关键代谢功能是由其微环境中的非实质细胞(NPC)控制的。我们培育出具有由人类肝细胞和NPC组成的人类肝组织的小鼠,其中包括人类免疫细胞、内皮细胞和星状细胞。人性化肝脏再现人类肝脏结构,执行重要的人类特有的代谢/稳态过程,并模拟人类病理学,包括纤维化和非酒精性脂肪肝病(NAFLD)。利用物种错配和脂质组学,我们证明人类 NPC 以旁分泌方式控制人类肝细胞的代谢功能。从机制上讲,我们发现了一种物种特异性相互作用,由窦内皮细胞分泌的 WNT2 通过受体 FZD5 控制肝细胞中的胆固醇摄取和胆汁酸结合。这些结果揭示了肝脏代谢的基本微环境调节及其人类特有的方面。
更新日期:2023-08-09
中文翻译:

人源化小鼠肝脏揭示内皮细胞对肝脏基本代谢功能的控制
肝细胞是人体的主要代谢中心,执行人类特有的功能,在人类疾病中改变,目前认为通过内分泌和细胞自主机制进行调节。在这里,我们表明人类肝细胞的关键代谢功能是由其微环境中的非实质细胞(NPC)控制的。我们培育出具有由人类肝细胞和NPC组成的人类肝组织的小鼠,其中包括人类免疫细胞、内皮细胞和星状细胞。人性化肝脏再现人类肝脏结构,执行重要的人类特有的代谢/稳态过程,并模拟人类病理学,包括纤维化和非酒精性脂肪肝病(NAFLD)。利用物种错配和脂质组学,我们证明人类 NPC 以旁分泌方式控制人类肝细胞的代谢功能。从机制上讲,我们发现了一种物种特异性相互作用,由窦内皮细胞分泌的 WNT2 通过受体 FZD5 控制肝细胞中的胆固醇摄取和胆汁酸结合。这些结果揭示了肝脏代谢的基本微环境调节及其人类特有的方面。































 京公网安备 11010802027423号
京公网安备 11010802027423号