当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Convergent Deboronative and Decarboxylative Phosphonylation Enabled by the Phosphite Radical Trap “BecaP”
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-08-08 , DOI: 10.1021/jacs.3c06524 Santosh K Pagire 1 , Chao Shu 1, 2 , Dominik Reich 1 , Adam Noble 1 , Varinder K Aggarwal 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-08-08 , DOI: 10.1021/jacs.3c06524 Santosh K Pagire 1 , Chao Shu 1, 2 , Dominik Reich 1 , Adam Noble 1 , Varinder K Aggarwal 1
Affiliation
|
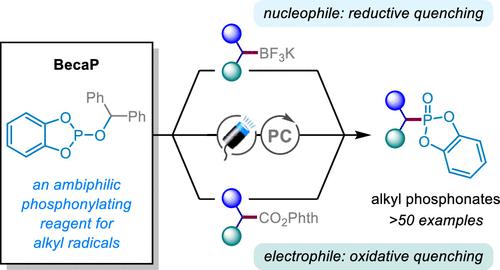
Carbon–phosphorus bond formation is significant in synthetic chemistry because phosphorus-containing compounds offer numerous indispensable biochemical roles. While there is a plethora of methods to access organophosphorus compounds, phosphonylations of readily accessible alkyl radicals to form aliphatic phosphonates are rare and not commonly used in synthesis. Herein, we introduce a novel phosphorus radical trap “BecaP” that enables facile and efficient phosphonylation of alkyl radicals under visible light photocatalytic conditions. Importantly, the ambiphilic nature of BecaP allows redox neutral reactions with both nucleophilic (activated by single-electron oxidation) and electrophilic (activated by single-electron reduction) alkyl radical precursors. Thus, a broad scope of feedstock alkyl potassium trifluoroborate salts and redox active carboxylate esters could be employed, with each class of substrate proceeding through a distinct mechanistic pathway. The mild conditions are applicable to the late-stage installation of phosphonate motifs into medicinal agents and natural products, which is showcased by the straightforward conversion of baclofen (muscle relaxant) to phaclofen (GABAB antagonist).
中文翻译:

亚磷酸盐自由基陷阱“BecaP”实现趋同脱硼和脱羧磷酸化
碳磷键的形成在合成化学中具有重要意义,因为含磷化合物具有许多不可或缺的生化作用。虽然有多种方法可以获取有机磷化合物,但将易于获取的烷基进行膦酰化以形成脂肪族膦酸酯的情况很少见,并且在合成中并不常用。在此,我们介绍了一种新型磷自由基捕获剂“BecaP”,它能够在可见光光催化条件下轻松有效地对烷基自由基进行磷酰化。重要的是,BecaP 的两亲性质允许与亲核(通过单电子氧化激活)和亲电(通过单电子还原激活)烷基自由基前体发生氧化还原中性反应。因此,可以使用多种原料烷基三氟硼酸钾盐和氧化还原活性羧酸酯,每一类底物都通过不同的机制途径进行。温和的条件适用于将膦酸酯基序安装到药物和天然产物中,这一点通过巴氯芬(肌肉松弛剂)直接转化为苯氯芬(GABA B 拮抗剂)来证明。
更新日期:2023-08-08
中文翻译:

亚磷酸盐自由基陷阱“BecaP”实现趋同脱硼和脱羧磷酸化
碳磷键的形成在合成化学中具有重要意义,因为含磷化合物具有许多不可或缺的生化作用。虽然有多种方法可以获取有机磷化合物,但将易于获取的烷基进行膦酰化以形成脂肪族膦酸酯的情况很少见,并且在合成中并不常用。在此,我们介绍了一种新型磷自由基捕获剂“BecaP”,它能够在可见光光催化条件下轻松有效地对烷基自由基进行磷酰化。重要的是,BecaP 的两亲性质允许与亲核(通过单电子氧化激活)和亲电(通过单电子还原激活)烷基自由基前体发生氧化还原中性反应。因此,可以使用多种原料烷基三氟硼酸钾盐和氧化还原活性羧酸酯,每一类底物都通过不同的机制途径进行。温和的条件适用于将膦酸酯基序安装到药物和天然产物中,这一点通过巴氯芬(肌肉松弛剂)直接转化为苯氯芬(GABA B 拮抗剂)来证明。































 京公网安备 11010802027423号
京公网安备 11010802027423号