SynOpen ( IF 2.0 ) Pub Date : 2023-08-08 , DOI: 10.1055/s-0040-1720079 Bathini Nagendra Babu 1, 2 , A. V. G. Prasanthi 1, 2
|
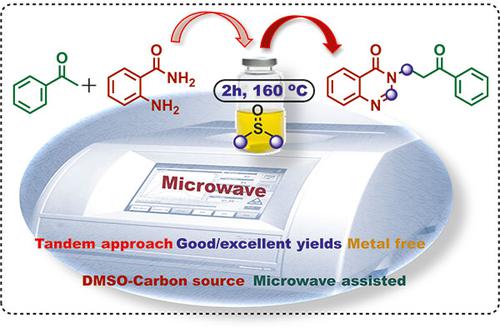
A convenient, time efficient, tandem approach for the synthesis of medicinally privileged 3-(3-oxo-3-arylpropyl) quinazolinones is developed from ubiquitously available acetophenones and anthranilamide via microwave irradiation. This transition-metal-free reaction is initiated by the oxidative annulation of anthranilamide and in situ generation of α,β-unsaturated carbonyl compounds from aryl ketones in the presence of K2S2O8 and dimethyl sulfoxide. The latter acts as a source of two carbons [methine (=CH–) and methylene (–CH2–)] apart from being the solvent. The reaction is carried out under microwave irradiation which has the advantage of homogenous heat distribution, reducing the reaction time drastically compared to the conventional heating reaction.
中文翻译:

DMSO 仲裁氧化环化后进行同系 N-烷基化:微波辅助高效且更环保的方法获取 3-(3-Oxo-3-芳基丙基) 喹唑啉酮
通过微波辐射,从普遍存在的苯乙酮和邻氨基苯甲酰胺中开发出一种方便、省时的串联方法,用于合成药用特殊的 3-(3-氧代-3-芳基丙基)喹唑啉酮。这种无过渡金属的反应是通过邻氨基苯甲酰胺的氧化成环以及在 K 2 S 2 O 8和二甲亚砜存在下由芳基酮原位生成 α,β-不饱和羰基化合物引发的。后者充当两个碳的来源[次甲基 (=CH–) 和亚甲基 (–CH 2–)]除了作为溶剂之外。该反应在微波辐射下进行,具有热量分布均匀的优点,与传统的加热反应相比,大大缩短了反应时间。































 京公网安备 11010802027423号
京公网安备 11010802027423号