当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Olefin Isomerization via Photoredox Catalytic Hydrogen Atom Transfer and Enantioselective Protonation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-08-08 , DOI: 10.1021/jacs.3c03732 Yang Liu 1 , Linghong Zhang 1 , Yong Zhang 1 , Shanshan Cao 2 , Xu Ban 2 , Yanli Yin 2, 3 , Xiaowei Zhao 1 , Zhiyong Jiang 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-08-08 , DOI: 10.1021/jacs.3c03732 Yang Liu 1 , Linghong Zhang 1 , Yong Zhang 1 , Shanshan Cao 2 , Xu Ban 2 , Yanli Yin 2, 3 , Xiaowei Zhao 1 , Zhiyong Jiang 1, 2
Affiliation
|
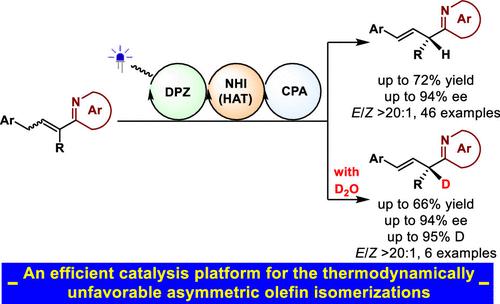
Asymmetric olefin isomerization can be appreciated as an ideal synthetic approach to access valuable enantioenriched C═C-containing molecules due to the excellent atom economy. Nonetheless, its occurrence usually requires a thermodynamic advantage, namely, a higher stability of the product to the substrate. It has thus led to rather limited examples of success. Herein, we report a photoredox catalytic hydrogen atom transfer (HAT) and enantioselective protonation strategy for the challenging asymmetric olefin isomerization. As a paradigm, by establishing a dual catalyst system involving a visible light photosensitizer DPZ and a chiral phosphoric acid, with the assistance of N-hydroxyimide to perform HAT, a wide array of allylic azaarene derivatives, featuring α-tertiary carbon stereocenters and β-C═C bonds, was synthesized with high yields, ees, and E/Z ratios starting from the conjugated α-substituted alkenylazaarene E/Z-mixtures. The good compatibility of assembling deuterium on stereocenters by using inexpensive D2O as a deuterium source further underscores the broad applicability and promising utility of this strategy. Moreover, mechanistic studies have provided clear insights into its challenges in terms of reactivity and enantioselectivity. The exploration will robustly inspire the development of thermodynamically unfavorable asymmetric olefin isomerizations.
中文翻译:

通过光氧化还原催化氢原子转移和对映选择性质子化进行不对称烯烃异构化
由于其优异的原子经济性,不对称烯烃异构化可被认为是获得有价值的对映体富集的含C=C分子的理想合成方法。尽管如此,它的发生通常需要热力学优势,即产物对基材具有更高的稳定性。因此,它的成功例子相当有限。在此,我们报告了用于具有挑战性的不对称烯烃异构化的光氧化还原催化氢原子转移(HAT)和对映选择性质子化策略。作为范例,通过建立可见光光敏剂DPZ和手性磷酸的双催化剂体系,在N-羟基酰亚胺的协助下进行HAT,得到一系列具有α-叔碳立构中心和β-碳立体中心的烯丙基氮杂芳烃衍生物。从共轭 α-取代的烯基氮杂芳烃E / Z混合物开始,以高产率、ee 和E / Z比率合成 C=C 键。使用廉价的D 2 O作为氘源在立体中心组装氘的良好相容性进一步强调了该策略的广泛适用性和有前景的实用性。此外,机理研究对其在反应性和对映选择性方面的挑战提供了清晰的见解。该探索将有力地激发热力学不利的不对称烯烃异构化的发展。
更新日期:2023-08-08
中文翻译:

通过光氧化还原催化氢原子转移和对映选择性质子化进行不对称烯烃异构化
由于其优异的原子经济性,不对称烯烃异构化可被认为是获得有价值的对映体富集的含C=C分子的理想合成方法。尽管如此,它的发生通常需要热力学优势,即产物对基材具有更高的稳定性。因此,它的成功例子相当有限。在此,我们报告了用于具有挑战性的不对称烯烃异构化的光氧化还原催化氢原子转移(HAT)和对映选择性质子化策略。作为范例,通过建立可见光光敏剂DPZ和手性磷酸的双催化剂体系,在N-羟基酰亚胺的协助下进行HAT,得到一系列具有α-叔碳立构中心和β-碳立体中心的烯丙基氮杂芳烃衍生物。从共轭 α-取代的烯基氮杂芳烃E / Z混合物开始,以高产率、ee 和E / Z比率合成 C=C 键。使用廉价的D 2 O作为氘源在立体中心组装氘的良好相容性进一步强调了该策略的广泛适用性和有前景的实用性。此外,机理研究对其在反应性和对映选择性方面的挑战提供了清晰的见解。该探索将有力地激发热力学不利的不对称烯烃异构化的发展。































 京公网安备 11010802027423号
京公网安备 11010802027423号