JACC: Heart Failure ( IF 10.3 ) Pub Date : 2023-08-07 , DOI: 10.1016/j.jchf.2023.05.026 Bertram Pitt 1 , Deepak L Bhatt 2 , Michael Szarek 3 , Christopher P Cannon 4 , Lawrence A Leiter 5 , Darren K McGuire 6 , Julia B Lewis 7 , Matthew C Riddle 8 , Adriaan A Voors 9 , Marco Metra 10 , Lars H Lund 11 , Michel Komajda 12 , Jeffrey M Testani 13 , Christopher S Wilcox 14 , Piotr Ponikowski 15 , Renato D Lopes 16 , Justin A Ezekowitz 17 , Franklin Sun 18 , Michael J Davies 19 , Subodh Verma 5 , Mikhail N Kosiborod 19 , Ph Gabriel Steg 20 ,
|
Background
Approximately 25% of patients admitted to hospitals for worsening heart failure (WHF) are readmitted within 30 days.
Objectives
The authors conducted a post hoc analysis of the SOLOIST-WHF (Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post-WHF) trial to evaluate the efficacy of sotagliflozin versus placebo to decrease mortality and HF-related events among patients who began study treatment on or before discharge from their index hospitalization.
Methods
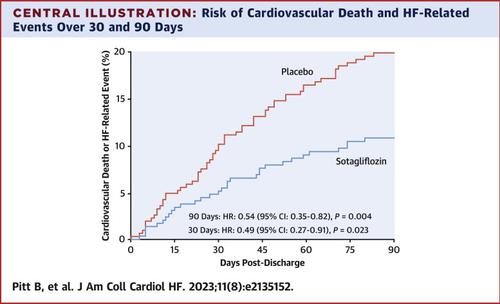
The main endpoint of interest was cardiovascular death or HF-related event (HF hospitalization or urgent care visit) occurring within 90 and 30 days after discharge for the index WHF hospitalization. Treatment comparisons were by proportional hazards models, generating HRs, 95% CIs, and P values.
Results
Of 1,222 randomized patients, 596 received study drug on or before their date of discharge. Sotagliflozin reduced the main endpoint at 90 days after discharge (HR: 0.54 [95% CI: 0.35-0.82]; P = 0.004) and at 30 days (HR: 0.49 [95% CI: 0.27-0.91]; P = 0.023) and all-cause mortality at 90 days (HR: 0.39 [95% CI: 0.17-0.88]; P = 0.024). In subgroup analyses, sotagliflozin reduced the 90-day main endpoint regardless of sex, age, estimated glomerular filtration rate, N-terminal pro-B-type natriuretic peptide, left ventricular ejection fraction, or mineralocorticoid receptor agonist use. Sotagliflozin was well-tolerated but with slightly higher rates of diarrhea and volume-related events than placebo.
Conclusions
Starting sotagliflozin before discharge in patients with type 2 diabetes hospitalized for WHF significantly decreased cardiovascular deaths and HF events through 30 and 90 days after discharge, emphasizing the importance of beginning sodium glucose cotransporter treatment before discharge.
中文翻译:

Sotagliflozin 对早期死亡率和心力衰竭相关事件的影响:SOLOIST-WHF 的事后分析
背景
大约 25% 因心力衰竭 (WHF) 恶化而入院的患者在 30 天内再次入院。
目标
作者对 SOLOIST-WHF(Sotagliflozin 对 WHF 后 2 型糖尿病患者心血管事件的影响)试验进行了事后分析,以评估 sotagliflozin 与安慰剂相比在降低开始接受治疗的患者中的死亡率和心力衰竭相关事件方面的功效。在出院时或出院前研究治疗。
方法
主要关注终点是出院后 90 和 30 天内发生的心血管死亡或 HF 相关事件(HF 住院或紧急护理就诊)。通过比例风险模型进行治疗比较,生成 HR、95% CI 和P值。
结果
在 1,222 名随机患者中,596 名患者在出院之日或之前接受了研究药物。索格列净降低了出院后 90 天的主要终点(HR:0.54 [95% CI:0.35-0.82];P = 0.004)和 30 天(HR:0.49 [95% CI:0.27-0.91];P = 0.023) 90 天时的全因死亡率(HR:0.39 [95% CI:0.17-0.88];P = 0.024)。在亚组分析中,无论性别、年龄、估计肾小球滤过率、N 末端 B 型利钠肽前体、左心室射血分数或盐皮质激素受体激动剂的使用情况如何,sotagliflozin 均降低了 90 天的主要终点。Sotagliflozin 的耐受性良好,但腹泻和容量相关事件的发生率略高于安慰剂。
结论
因 WHF 住院的 2 型糖尿病患者在出院前开始使用 sotagliflozin 可显着降低出院后 30 和 90 天的心血管死亡和心力衰竭事件,这强调了出院前开始钠葡萄糖协同转运蛋白治疗的重要性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号