Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fluorescence sensing and adsorption kinetics of Gd-doped AgInS2 I-III-VI quantum dots – A case study of Ag+ ions interactions
Heliyon ( IF 3.4 ) Pub Date : 2023-08-07 , DOI: 10.1016/j.heliyon.2023.e19020
Bambesiwe M May 1, 2 , Olayemi J Fakayode 1 , Mokae F Bambo 2 , Ajay K Mishra 3, 4 , Edward N Nxumalo 1
Heliyon ( IF 3.4 ) Pub Date : 2023-08-07 , DOI: 10.1016/j.heliyon.2023.e19020
Bambesiwe M May 1, 2 , Olayemi J Fakayode 1 , Mokae F Bambo 2 , Ajay K Mishra 3, 4 , Edward N Nxumalo 1
Affiliation
|
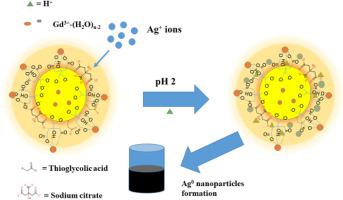
The poor fluorescence properties of magneto-fluorescent paramagnetic-ion (Gd, Mn, or Co) doped I-III-VI quantum dots (QDs) at higher paramagnetic-ion doping concentrations have limited their use in magnetic-driven water-based applications. This work presents, for the first time, the use of stable magneto-fluorescent Gd-doped AgInS2 QDs at high Gd mole ratios of 16, 20, and 30 for the fluorescence detection and adsorption of Ag+ ions in water environments. The effect of pH, initial concentration, contact time, and adsorbent dosage were systematically evaluated. The AgInS2 QDs with the least Gd mole ratio (16) exhibited the best fluorescence characteristics (LOD = 0.88, R2 = 0.9549) while all materials showed good adsorption properties under optimized conditions (pH of 2, initial concentration of 30 ppm, contact time of 10 min and adsorbent dosage of 0.02 g) and a pseudo 2nd order reaction was followed. The adsorption mechanism was proposed to be a combination of ion-exchange, electrostatic interaction, complexation, and diffusion processes. Application in environmental wastewater samples revealed complete removal of Ag + ions alongside Ti2+ Pb2+ , Ni2+ , Cr3+ , and Zn2+ ions.
中文翻译:

Gd 掺杂 AgInS2 I-III-VI 量子点的荧光传感和吸附动力学——Ag+ 离子相互作用的案例研究
磁荧光顺磁离子(Gd、Mn 或 Co)掺杂的 I-III-VI 量子点 (QD) 在较高的顺磁离子掺杂浓度下荧光性能较差,这限制了它们在磁驱动水基应用中的使用。这项工作首次提出了使用 16、20 和 30 高 Gd 摩尔比的稳定磁荧光 Gd 掺杂 AgInS2 QDs 在水环境中对 Ag+ 离子进行荧光检测和吸附。系统评价了 pH 值、初始浓度、接触时间和吸附剂用量的影响。Gd 摩尔比最小 (16) 的 AgInS2 量子点表现出最佳的荧光特性 (LOD = 0.88,R2 = 0.9549),而所有材料在最佳条件下 (pH 值为 2,初始浓度为 30 ppm,接触时间为 10 min,吸附剂用量为 0.02 g) 均表现出良好的吸附性能,并遵循伪 2 级反应。吸附机制被认为是离子交换、静电相互作用、络合和扩散过程的组合。在环境废水样品中的应用显示,完全去除了 Ag + 离子以及 Ti2+ Pb2+、Ni2+、Cr3+ 和 Zn2+ 离子。
更新日期:2023-08-07
中文翻译:

Gd 掺杂 AgInS2 I-III-VI 量子点的荧光传感和吸附动力学——Ag+ 离子相互作用的案例研究
磁荧光顺磁离子(Gd、Mn 或 Co)掺杂的 I-III-VI 量子点 (QD) 在较高的顺磁离子掺杂浓度下荧光性能较差,这限制了它们在磁驱动水基应用中的使用。这项工作首次提出了使用 16、20 和 30 高 Gd 摩尔比的稳定磁荧光 Gd 掺杂 AgInS2 QDs 在水环境中对 Ag+ 离子进行荧光检测和吸附。系统评价了 pH 值、初始浓度、接触时间和吸附剂用量的影响。Gd 摩尔比最小 (16) 的 AgInS2 量子点表现出最佳的荧光特性 (LOD = 0.88,R2 = 0.9549),而所有材料在最佳条件下 (pH 值为 2,初始浓度为 30 ppm,接触时间为 10 min,吸附剂用量为 0.02 g) 均表现出良好的吸附性能,并遵循伪 2 级反应。吸附机制被认为是离子交换、静电相互作用、络合和扩散过程的组合。在环境废水样品中的应用显示,完全去除了 Ag + 离子以及 Ti2+ Pb2+、Ni2+、Cr3+ 和 Zn2+ 离子。

































 京公网安备 11010802027423号
京公网安备 11010802027423号