当前位置:
X-MOL 学术
›
J. Ethnopharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A blend of Withania somnifera (L.) Dunal root and Abelmoschus esculentus (L.) Moench fruit extracts relieves constipation and improves bowel function: A proof-of-concept clinical investigation
Journal of Ethnopharmacology ( IF 4.8 ) Pub Date : 2023-08-04 , DOI: 10.1016/j.jep.2023.116997 Raghu Sarath Punukollu 1 , Arun Kumar Chadalawada 2 , Kalyani Siddabattuni 2 , Naga Tejaswi Gogineni 3
Journal of Ethnopharmacology ( IF 4.8 ) Pub Date : 2023-08-04 , DOI: 10.1016/j.jep.2023.116997 Raghu Sarath Punukollu 1 , Arun Kumar Chadalawada 2 , Kalyani Siddabattuni 2 , Naga Tejaswi Gogineni 3
Affiliation
|
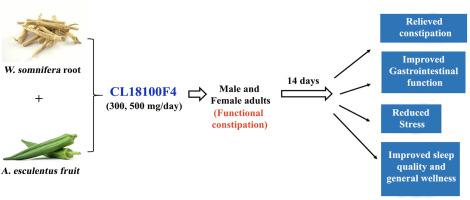
(L.) Dunal (WS) and (L.) Moench (AE) are known as Ashwagandha and Okra, respectively, important herbs in traditional medicine for their diverse therapeutic values. WS root is an adaptogen that relieves stress and anxiety and promotes sleep. AE fruit or Okra is widely consumed as a vegetable and is traditionally used to treat diabetes, gastric irritations, ulcers, and obesity. The present randomized, double-blind, placebo-controlled study aimed to establish a proof-of-concept evaluating the efficacy and tolerability of a proprietary blend of standardized extracts of WS root and AE fruit, CL18100F4 in relieving constipation and improving quality of life in adults. Forty-eight male and female participants (age: 25–60 years) with functional constipation (following Rome-III criteria) were randomized into placebo, 300 or 500 mg of CL18100F4 groups, and supplemented for fourteen consecutive days. CL18100F4 supplementation significantly ( < 0.0001) reduced the Patient Assessment of Constipation-Symptoms (PAC-SYM), Patient Assessment of Constipation-Quality of Life (PAC-QOL), and Gastrointestinal Symptom Rating Scale (GSRS) scores. CL18100F4 supplementation improved sleep quality and reduced stress (p < 0.0001). At the end of the study, CL18100F4-500 subjects showed significant increases in serum serotonin, gastrin, and interleukin-10 and decrease in interleukin-6 and cortisol levels. Participants’ hematology, total blood chemistry, vital signs, and urinalysis parameters were within the normal ranges. No adverse events were reported. This short-duration, single-site clinical investigation demonstrates that CL18100F4 supplementation is tolerable, helps relieve constipation, reduces stress, and improves gastrointestinal function, sleep quality, and general wellness in adults. Clinical Trials Registry- India (CTRI/2020/11/029320); Registered on 24/11/2020. Available at: .
中文翻译:

Withania somnifera (L.) Dunal 根和 Abelmoschus esculentus (L.) Moench 果实提取物的混合物可缓解便秘并改善肠道功能:概念验证临床研究
(L.) Dunal (WS) 和 (L.) Moench (AE) 分别被称为 Ashwagandha 和 Okra,因其多种治疗价值而成为传统医学中的重要草药。 WS root 是一种适应原,可以缓解压力和焦虑并促进睡眠。 AE 水果或秋葵作为蔬菜被广泛食用,传统上用于治疗糖尿病、胃刺激、溃疡和肥胖。本随机、双盲、安慰剂对照研究旨在建立概念验证,评估 WS 根和 AE 果实标准化提取物的专有混合物 CL18100F4 在缓解便秘和改善生活质量方面的功效和耐受性。成年人。患有功能性便秘(遵循罗马 III 标准)的 48 名男性和女性参与者(年龄:25-60 岁)被随机分为安慰剂组、300 或 500 毫克 CL18100F4 组,并连续补充十四天。补充 CL18100F4 显着降低 (< 0.0001) 患者便秘症状评估 (PAC-SYM)、患者便秘症状评估 (PAC-QOL) 和胃肠道症状评定量表 (GSRS) 评分。补充 CL18100F4 可改善睡眠质量并减轻压力 (p < 0.0001)。研究结束时,CL18100F4-500 受试者的血清血清素、胃泌素和白细胞介素 10 显着增加,而白细胞介素 6 和皮质醇水平显着下降。参与者的血液学、全血化学、生命体征和尿液分析参数均在正常范围内。没有不良事件的报告。这项短期、单中心临床研究表明,CL18100F4 补充剂是可耐受的,有助于缓解成人便秘、减轻压力,并改善胃肠功能、睡眠质量和总体健康状况。 临床试验注册中心 - 印度 (CTRI/2020/11/029320);注册于 2020 年 11 月 24 日。可以在: 。
更新日期:2023-08-04
中文翻译:

Withania somnifera (L.) Dunal 根和 Abelmoschus esculentus (L.) Moench 果实提取物的混合物可缓解便秘并改善肠道功能:概念验证临床研究
(L.) Dunal (WS) 和 (L.) Moench (AE) 分别被称为 Ashwagandha 和 Okra,因其多种治疗价值而成为传统医学中的重要草药。 WS root 是一种适应原,可以缓解压力和焦虑并促进睡眠。 AE 水果或秋葵作为蔬菜被广泛食用,传统上用于治疗糖尿病、胃刺激、溃疡和肥胖。本随机、双盲、安慰剂对照研究旨在建立概念验证,评估 WS 根和 AE 果实标准化提取物的专有混合物 CL18100F4 在缓解便秘和改善生活质量方面的功效和耐受性。成年人。患有功能性便秘(遵循罗马 III 标准)的 48 名男性和女性参与者(年龄:25-60 岁)被随机分为安慰剂组、300 或 500 毫克 CL18100F4 组,并连续补充十四天。补充 CL18100F4 显着降低 (< 0.0001) 患者便秘症状评估 (PAC-SYM)、患者便秘症状评估 (PAC-QOL) 和胃肠道症状评定量表 (GSRS) 评分。补充 CL18100F4 可改善睡眠质量并减轻压力 (p < 0.0001)。研究结束时,CL18100F4-500 受试者的血清血清素、胃泌素和白细胞介素 10 显着增加,而白细胞介素 6 和皮质醇水平显着下降。参与者的血液学、全血化学、生命体征和尿液分析参数均在正常范围内。没有不良事件的报告。这项短期、单中心临床研究表明,CL18100F4 补充剂是可耐受的,有助于缓解成人便秘、减轻压力,并改善胃肠功能、睡眠质量和总体健康状况。 临床试验注册中心 - 印度 (CTRI/2020/11/029320);注册于 2020 年 11 月 24 日。可以在: 。































 京公网安备 11010802027423号
京公网安备 11010802027423号