Cellular Signalling ( IF 4.4 ) Pub Date : 2023-08-05 , DOI: 10.1016/j.cellsig.2023.110837 Xingming Zhang 1 , Lou Geng 1 , Li Yang 2 , Yingying Wang 2 , Zhihui Zou 2 , Youping Zhang 2 , Hanzhang Xu 2 , Hu Lei 2 , Yang Cao 3 , Yingli Wu 2 , Wenli Gu 1 , Li Zhou 4
|
Background
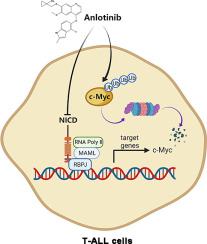
Despite some progress having been made regarding the treatment of T-cell acute lymphoblastic leukemia (T-ALL), the prognosis of T-ALL, particularly adult T-ALL, is still poor. Identifying novel, effective anti-T-ALL drugs is of great significance. Anlotinib, an oral tyrosine kinase inhibitor currently utilized in the treatment of lung cancer, exhibited a promising anti-T-ALL effect. A comprehensive study should therefore be conducted to explore both the in vitro as well as in vivo mechanisms of the anti-T-ALL effects of anlotinib.
Methods
CCK8 assays and flow cytometry were employed to investigate the viability, cell cycle distribution, and apoptosis of T-ALL cell lines when treated with anlotinib. T-ALL xenograft mouse models were established to examine the in vivo antileukemic effects of anlotinib. Cellular and molecular analysis of T-ALL were conducted to define the underlying mechanisms.
Results
In vitro, anlotinib significantly inhibited the viability, induced G2/M phase arrest and apoptosis in T-ALL cell lines in a concentration-dependent pattern. In vivo, anlotinib also demonstrated a strong anti-tumor effect at doses that are well-tolerated. Interestingly, anlotinib could decrease the protein levels of the intracellular domains of NOTCH1 (ICN1) and c-Myc, two important targets for T-ALL. Mechanistically, anlotinib-induced c-Myc reduction was associated with proteasome-mediated degradation, while the ICN1 reduction was not due to protein degradation or transcriptional repression.
Conclusions
The present study showed that anlotinib may be a promising anti-T-ALL candidate drug, and simultaneous reduction of the protein levels of both ICN1 and c-Myc may contribute to the anti-T-ALL efficacy of anlotinib.
中文翻译:

安罗替尼在体外和体内发挥抗 T 细胞急性淋巴细胞白血病作用
背景
尽管T细胞急性淋巴细胞白血病(T-ALL)的治疗取得了一些进展,但T-ALL,特别是成人T-ALL的预后仍然很差。寻找新型、有效的抗T-ALL药物具有重要意义。安罗替尼是一种口服酪氨酸激酶抑制剂,目前用于治疗肺癌,表现出良好的抗 T-ALL 作用。因此,应进行全面的研究来探索安罗替尼抗 T-ALL 作用的体外和体内机制。
方法
采用 CCK8 检测和流式细胞术研究安罗替尼处理后 T-ALL 细胞系的活力、细胞周期分布和凋亡。建立T-ALL异种移植小鼠模型来检查安罗替尼的体内抗白血病作用。对 T-ALL 进行细胞和分子分析以确定其潜在机制。
结果
在体外,安罗替尼以浓度依赖性模式显着抑制 T-ALL 细胞系的活力,诱导 G2/M 期停滞和凋亡。在体内,安罗替尼在耐受良好的剂量下也表现出强大的抗肿瘤作用。有趣的是,安罗替尼可以降低 T-ALL 的两个重要靶点 NOTCH1 (ICN1) 和 c-Myc 细胞内结构域的蛋白质水平。从机制上讲,安罗替尼诱导的 c-Myc 减少与蛋白酶体介导的降解有关,而 ICN1 减少并非由于蛋白质降解或转录抑制。
结论
本研究表明,安罗替尼可能是一种有前途的抗T-ALL候选药物,同时降低ICN1和c-Myc的蛋白水平可能有助于安罗替尼的抗T-ALL疗效。






























 京公网安备 11010802027423号
京公网安备 11010802027423号