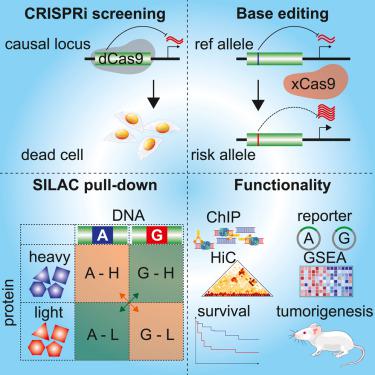
全基因组关联研究以及表达数量性状基因座 (eQTL) 作图已经确定了前列腺癌 (PCa) 中的数百个单核苷酸多态性 (SNP) 及其靶基因,但这些风险基因座的功能表征仍然具有挑战性。为了筛选潜在的调节性 SNP,我们设计了一个包含 9,133 个指导 RNA (gRNA) 的 CRISPRi 文库,覆盖 PCa 中涉及的 2,166 个候选 SNP 位点,并鉴定了 117 个 SNP,它们可以调节 90 个基因,从而发挥 PCa 细胞生长优势。其中,rs60464856 被筛选中显着耗尽的多个 gRNA 覆盖(FDR < 0.05)。 PRACTICAL 和 FinnGen 队列中的汇总 SNP 关联分析显示 rs60464856 G 等位基因的 PCa 风险显着较高(p 值分别 = 1.2 × 10 -16和 3.2 × 10 -7 )。随后的 eQTL 分析表明,G 等位基因与多个数据集中RUVBL1表达增加相关。进一步的 CRISPRi 和 xCas9 碱基编辑证实 rs60464856 G 等位基因导致RUVBL1表达升高。此外,基于 SILAC 的蛋白质组学分析证明了粘连蛋白亚基在 rs60464856 区域的等位基因结合,其中 HiC 数据集显示前列腺细胞系中一致的染色质相互作用。在异种移植小鼠模型中, RUVBL1缺失抑制 PCa 细胞增殖和肿瘤生长。基因集富集分析表明RUVBL1表达与细胞周期相关途径之间存在关联。在 TCGA 数据集中, RUVBL1表达增加和细胞周期通路激活与 PCa 存活率低相关。 我们的 CRISPRi 筛选优先考虑了大约一百个对前列腺细胞增殖至关重要的调节 SNP。结合蛋白质组学和功能研究,我们描述了 rs60464856 和RUVBL1在 PCa 进展中的机制作用。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Combined CRISPRi and proteomics screening reveal a cohesin-CTCF-bound allele contributing to increased expression of RUVBL1 and prostate cancer progression
Genome-wide association studies along with expression quantitative trait locus (eQTL) mapping have identified hundreds of single-nucleotide polymorphisms (SNPs) and their target genes in prostate cancer (PCa), yet functional characterization of these risk loci remains challenging. To screen for potential regulatory SNPs, we designed a CRISPRi library containing 9,133 guide RNAs (gRNAs) to cover 2,166 candidate SNP loci implicated in PCa and identified 117 SNPs that could regulate 90 genes for PCa cell growth advantage. Among these, rs60464856 was covered by multiple gRNAs significantly depleted in screening (FDR < 0.05). Pooled SNP association analysis in the PRACTICAL and FinnGen cohorts showed significantly higher PCa risk for the rs60464856 G allele (p value = 1.2 × 10−16 and 3.2 × 10−7, respectively). Subsequent eQTL analysis revealed that the G allele is associated with increased RUVBL1 expression in multiple datasets. Further CRISPRi and xCas9 base editing confirmed that the rs60464856 G allele leads to elevated RUVBL1 expression. Furthermore, SILAC-based proteomic analysis demonstrated allelic binding of cohesin subunits at the rs60464856 region, where the HiC dataset showed consistent chromatin interactions in prostate cell lines. RUVBL1 depletion inhibited PCa cell proliferation and tumor growth in a xenograft mouse model. Gene-set enrichment analysis suggested an association of RUVBL1 expression with cell-cycle-related pathways. Increased expression of RUVBL1 and activation of cell-cycle pathways were correlated with poor PCa survival in TCGA datasets. Our CRISPRi screening prioritized about one hundred regulatory SNPs essential for prostate cell proliferation. In combination with proteomics and functional studies, we characterized the mechanistic role of rs60464856 and RUVBL1 in PCa progression.






















































 京公网安备 11010802027423号
京公网安备 11010802027423号