Talanta ( IF 5.6 ) Pub Date : 2023-08-03 , DOI: 10.1016/j.talanta.2023.125028 Lei Wen 1 , Mengqi Shao 2 , Yinhui Li 1 , Yanjun Zhang 3 , Chao Peng 1 , Huan Yu 2 , Kai Zhang 3
|
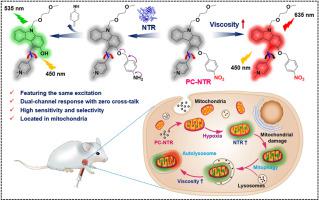
Mitophagy is an essential physiological process that eliminates damaged mitochondria via lysosomes. It is reported that hypoxia, inflammatory stimuli or other stress conditions could lead to mitochondrial damage and mitochondrial dysfunction, which induces the process of mitophagy. Herein, we report a novel fluorescent probe PC-NTR for imaging hypoxia-induced mitophagy by monitoring the change of nitroreductase and viscosity simultaneously. To our delight, PC-NTR could respond simultaneously to nitroreductase and viscosity at different fluorescence channels with no mutual interference under the same excitation wavelength. The fluorescence emission around 535 nm was enhanced dramatically after addition of nitroreductase while the fluorescence emission around 635 nm heightened as the viscosity increased. The probe would be able to selectively targeting of mitochondria in cells because of the positively charged pyridine salt structure of PC-NTR. The probe was successfully applied to assess the different levels of hypoxia and real-time imaging of mitochondrial autophagy in live cells. More importantly, using dual channel imaging, PC-NTR could be used to distinguish cancer cells from normal cells and was successfully applied to imaging experiments in HeLa-derived tumor-bearing nude mice. Therefore, PC-NTR would be an important molecular tool for hypoxia imaging and detecting solid tumors in vivo.
中文翻译:

通过同一激发下NTR和粘度的双通道实时成像揭示缺氧诱导的线粒体自噬过程
线粒体自噬是通过溶酶体消除受损线粒体的重要生理过程。据报道,缺氧、炎症刺激或其他应激条件可导致线粒体损伤和线粒体功能障碍,从而诱发线粒体自噬过程。在此,我们报道了一种新型荧光探针PC-NTR,通过同时监测硝基还原酶和粘度的变化来对缺氧诱导的线粒体自噬进行成像。令我们高兴的是,PC-NTR可以在不同的荧光通道上同时响应硝基还原酶和粘度,并且在相同的激发波长下不会相互干扰。添加硝基还原酶后,535 nm 附近的荧光发射显着增强,而 635 nm 附近的荧光发射随着粘度的增加而增强。由于 PC-NTR 的带正电荷的吡啶盐结构,该探针能够选择性地靶向细胞中的线粒体。该探针成功应用于评估活细胞中不同缺氧水平和线粒体自噬的实时成像。更重要的是,利用双通道成像,PC-NTR可用于区分癌细胞与正常细胞,并成功应用于HeLa来源的荷瘤裸鼠的成像实验。因此,PC-NTR将成为缺氧成像和体内实体瘤检测的重要分子工具。































 京公网安备 11010802027423号
京公网安备 11010802027423号