Molecular Cell ( IF 14.5 ) Pub Date : 2023-08-02 , DOI: 10.1016/j.molcel.2023.07.006 Hongshan Zhang 1 , Zhubing Shi 2 , Edward J Banigan 3 , Yoori Kim 4 , Hongtao Yu 2 , Xiao-Chen Bai 5 , Ilya J Finkelstein 1
|
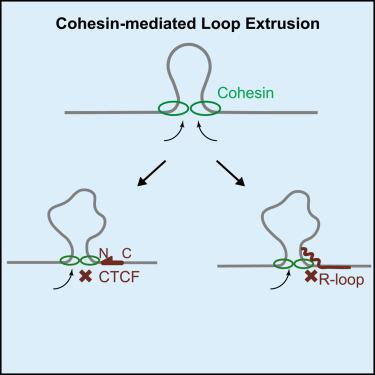
Cohesin and CCCTC-binding factor (CTCF) are key regulatory proteins of three-dimensional (3D) genome organization. Cohesin extrudes DNA loops that are anchored by CTCF in a polar orientation. Here, we present direct evidence that CTCF binding polarity controls cohesin-mediated DNA looping. Using single-molecule imaging, we demonstrate that a critical N-terminal motif of CTCF blocks cohesin translocation and DNA looping. The cryo-EM structure of the cohesin-CTCF complex reveals that this CTCF motif ahead of zinc fingers can only reach its binding site on the STAG1 cohesin subunit when the N terminus of CTCF faces cohesin. Remarkably, a C-terminally oriented CTCF accelerates DNA compaction by cohesin. DNA-bound Cas9 and Cas12a ribonucleoproteins are also polar cohesin barriers, indicating that stalling may be intrinsic to cohesin itself. Finally, we show that RNA-DNA hybrids (R-loops) block cohesin-mediated DNA compaction in vitro and are enriched with cohesin subunits in vivo, likely forming TAD boundaries.
中文翻译:

CTCF 和 R 环是粘连蛋白介导的 DNA 环的边界
粘连蛋白和 CCCTC 结合因子 (CTCF) 是三维 (3D) 基因组组织的关键调节蛋白。 Cohesin 挤出由 CTCF 以极性方向锚定的 DNA 环。在这里,我们提供了 CTCF 结合极性控制粘连蛋白介导的 DNA 环的直接证据。使用单分子成像,我们证明 CTCF 的关键 N 末端基序可阻断粘连蛋白易位和 DNA 成环。粘连蛋白-CTCF复合物的冷冻电镜结构表明,当CTCF的N末端面向粘连蛋白时,锌指前面的CTCF基序只能到达其在STAG1粘连蛋白亚基上的结合位点。值得注意的是,C 端定向的 CTCF 通过粘连蛋白加速 DNA 压缩。 DNA 结合的 Cas9 和 Cas12a 核糖核蛋白也是极性粘连蛋白屏障,表明停滞可能是粘连蛋白本身固有的。最后,我们发现 RNA-DNA 杂合体(R 环)在体外阻断粘连蛋白介导的 DNA 压缩,并在体内富含粘连蛋白亚基,可能形成 TAD 边界。































 京公网安备 11010802027423号
京公网安备 11010802027423号