Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-08-01 , DOI: 10.1016/j.cej.2023.145146
Zhengshan Yang , Huayi Yin , Bowen Deng , Dihua Wang
|
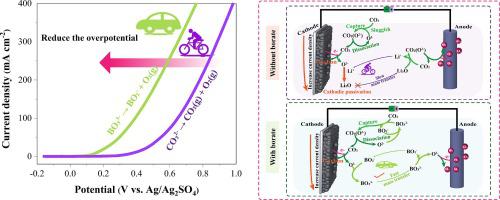
Molten carbonate electrolysis cells (MCEC) are a promising electrochemical CO2 capture and conversion process. However, the CO2 absorption and energy efficiencies are limited by the sluggish CO2 absorption kinetics and high electrode overpotentials. Herein, we propose an electrolyte engineering strategy to improve the CO2 absorption rate and reduce overpotentials at both the anode and the cathode. When BO33− was added into molten Li2CO3-Na2CO3-K2CO3, the CO2 adsorption efficiency was improved from 3.3% to 60%, the overpotential was reduced by ∼ 567 mV at the cathode and by ∼ 238 mV at the anode under 200 mA cm−2. Accordingly, the energy efficiency reached 76.2 % at 650 °C. The improved CO2 absorption and energy efficiencies are thanks to the BO33− that changes the thermodynamic properties of the molten carbonate, i.e., the BO33−–CO32− complex reduces the energy barrier for the reduction of CO32− at the cathode and for the liberation of O2− that can be oxidized at a lower potential than CO32− at the anode. Therefore, the electrolyte engineering is an effective strategy for designing high-temperature CO2 electrolyzers with high CO2 absorption and energy efficiency.
中文翻译:

CO2 高效熔融碳酸盐电解的电解质工程
熔融碳酸盐电解池(MCEC)是一种很有前景的电化学CO 2捕获和转化过程。然而,CO 2吸收和能量效率受到缓慢的CO 2吸收动力学和高电极过电势的限制。在此,我们提出了一种电解质工程策略来提高CO 2吸收率并降低阳极和阴极的过电势。当BO 3 3−添加到熔融的Li 2 CO 3 -Na 2 CO 3 -K 2 CO 3中时,CO 2在200 mA cm -2下,吸附效率从3.3%提高到60%,阴极过电势降低约567 mV,阳极过电势降低约238 mV 。因此,在650℃时能量效率达到76.2%。CO 2吸收和能量效率的提高归功于BO 3 3−改变了熔融碳酸盐的热力学性质,即BO 3 3− –CO 3 2−络合物降低了CO 3 2还原的能量势垒−在阴极释放 O 2− ,其可以在比 CO 3 2−更低的电势下被氧化在阳极。因此,电解质工程是设计具有高CO 2吸收和能源效率的高温CO 2电解槽的有效策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号