当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulating On-Demand Release of Vancomycin from Implant Coatings via Chemical Modification of a Micrococcal Nuclease-Sensitive Oligonucleotide Linker
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-07-31 , DOI: 10.1021/acsami.3c05881
Jordan D Skelly 1 , Feiyang Chen 1 , Shing-Yun Chang 1 , Rewati R Ujjwal 1 , Ananta Ghimire 1 , David C Ayers 1 , Jie Song 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2023-07-31 , DOI: 10.1021/acsami.3c05881
Jordan D Skelly 1 , Feiyang Chen 1 , Shing-Yun Chang 1 , Rewati R Ujjwal 1 , Ananta Ghimire 1 , David C Ayers 1 , Jie Song 1, 2
Affiliation
|
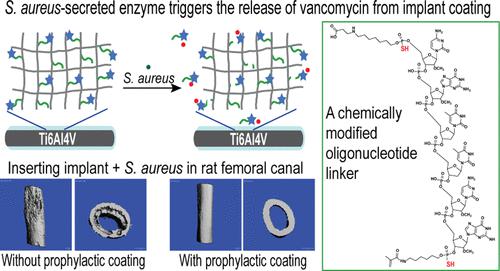
Periprosthetic infections are one of the most serious complications in orthopedic surgeries, and those caused by Staphylococcus aureus (S. aureus) are particularly hard to treat due to their tendency to form biofilms on implants and their notorious ability to invade the surrounding bones. The existing prophylactic local antibiotic deliveries involve excessive drug loading doses that could risk the development of drug resistance strains. Utilizing an oligonucleotide linker sensitive to micrococcal nuclease (MN) cleavage, we previously developed an implant coating capable of releasing covalently tethered vancomycin, triggered by S. aureus-secreted MN, to prevent periprosthetic infections in the mouse intramedullary (IM) canal. To further engineer this exciting platform to meet broader clinical needs, here, we chemically modified the oligonucleotide linker by a combination of 2′-O-methylation and phosphorothioate modification to achieve additional modulation of its stability/sensitivity to MN and the kinetics of MN-triggered on-demand release. We found that when all phosphodiester bonds within the oligonucleotide linker 5′-carboxy-mCmGTTmCmG-3-acrydite, except for the one between TT, were replaced by phosphorothioate, the oligonucleotide (6PS) stability significantly increased and enabled the most sustained release of tethered vancomycin from the coating. By contrast, when only the peripheral phosphodiester bonds at the 5′- and 3′-ends were replaced by phosphorothioate, the resulting oligonucleotide (2PS) linker was cleaved by MN more rapidly than that without any PS modifications (0PS). Using a rat femoral canal periprosthetic infection model where 1000 CFU S. aureus was inoculated at the time of IM pin insertion, we showed that the prophylactic implant coating containing either 0PS- or 2PS-modified oligonucleotide linker effectively eradicated the bacteria by enabling the rapid on-demand release of vancomycin. No bacteria were detected from the explanted pins, and no signs of cortical bone changes were detected in these treatment groups throughout the 3 month follow-ups. With an antibiotic tethering dose significantly lower than conventional antibiotic-bearing bone cements, these coatings also exhibited excellent biocompatibility. These chemically modified oligonucleotides could help tailor prophylactic anti-infective coating strategies to meet a range of clinical challenges where the risks for S. aureus prosthetic infections range from transient to long-lasting.
中文翻译:

通过微球菌核酸酶敏感寡核苷酸连接体的化学修饰调节万古霉素从植入物涂层的按需释放
假体周围感染是骨科手术中最严重的并发症之一,由金黄色葡萄球菌( S. aureus ) 引起的感染特别难以治疗,因为它们容易在植入物上形成生物膜,并且具有众所周知的侵入周围骨骼的能力。现有的预防性局部抗生素给药涉及过量的载药剂量,可能存在产生耐药菌株的风险。利用对微球菌核酸酶 (MN) 裂解敏感的寡核苷酸接头,我们之前开发了一种植入物涂层,能够在金黄色葡萄球菌分泌的 MN 触发下释放共价束缚的万古霉素,以预防小鼠髓内 (IM) 管的假体周围感染。为了进一步设计这个令人兴奋的平台以满足更广泛的临床需求,在这里,我们通过 2'-O-甲基化和硫代磷酸酯修饰的组合对寡核苷酸接头进行化学修饰,以实现其对 MN 的稳定性/敏感性以及 MN- 动力学的额外调节。触发按需发布。我们发现,当寡核苷酸接头 5'-羧基-mCmGTTmCmG-3-acrydite 内的所有磷酸二酯键(除了 TT 之间的磷酸二酯键)都被硫代磷酸酯取代时,寡核苷酸 (6PS) 的稳定性显着增加,并能够最持久地释放所束缚的物质。涂层中的万古霉素。相比之下,当仅 5' 端和 3' 端的外围磷酸二酯键被硫代磷酸酯取代时,所得寡核苷酸 (2PS) 连接体被 MN 切割的速度比没有任何 PS 修饰的连接体 (0PS) 更快。使用大鼠股骨管假体周围感染模型,其中 1000 CFU S. 在 IM 针插入时接种金黄色葡萄球菌,我们发现含有 0PS 或 2PS 修饰寡核苷酸接头的预防性植入物涂层可通过快速按需释放万古霉素来有效根除细菌。在 3 个月的随访期间,这些治疗组中没有检测到外植针中的细菌,也没有检测到皮质骨变化的迹象。由于抗生素束缚剂量显着低于传统的含抗生素骨水泥,这些涂层还表现出优异的生物相容性。这些化学修饰的寡核苷酸可以帮助定制预防性抗感染涂层策略,以满足一系列临床挑战,其中金黄色葡萄球菌假体感染的风险从短暂到长期不等。
更新日期:2023-07-31
中文翻译:

通过微球菌核酸酶敏感寡核苷酸连接体的化学修饰调节万古霉素从植入物涂层的按需释放
假体周围感染是骨科手术中最严重的并发症之一,由金黄色葡萄球菌( S. aureus ) 引起的感染特别难以治疗,因为它们容易在植入物上形成生物膜,并且具有众所周知的侵入周围骨骼的能力。现有的预防性局部抗生素给药涉及过量的载药剂量,可能存在产生耐药菌株的风险。利用对微球菌核酸酶 (MN) 裂解敏感的寡核苷酸接头,我们之前开发了一种植入物涂层,能够在金黄色葡萄球菌分泌的 MN 触发下释放共价束缚的万古霉素,以预防小鼠髓内 (IM) 管的假体周围感染。为了进一步设计这个令人兴奋的平台以满足更广泛的临床需求,在这里,我们通过 2'-O-甲基化和硫代磷酸酯修饰的组合对寡核苷酸接头进行化学修饰,以实现其对 MN 的稳定性/敏感性以及 MN- 动力学的额外调节。触发按需发布。我们发现,当寡核苷酸接头 5'-羧基-mCmGTTmCmG-3-acrydite 内的所有磷酸二酯键(除了 TT 之间的磷酸二酯键)都被硫代磷酸酯取代时,寡核苷酸 (6PS) 的稳定性显着增加,并能够最持久地释放所束缚的物质。涂层中的万古霉素。相比之下,当仅 5' 端和 3' 端的外围磷酸二酯键被硫代磷酸酯取代时,所得寡核苷酸 (2PS) 连接体被 MN 切割的速度比没有任何 PS 修饰的连接体 (0PS) 更快。使用大鼠股骨管假体周围感染模型,其中 1000 CFU S. 在 IM 针插入时接种金黄色葡萄球菌,我们发现含有 0PS 或 2PS 修饰寡核苷酸接头的预防性植入物涂层可通过快速按需释放万古霉素来有效根除细菌。在 3 个月的随访期间,这些治疗组中没有检测到外植针中的细菌,也没有检测到皮质骨变化的迹象。由于抗生素束缚剂量显着低于传统的含抗生素骨水泥,这些涂层还表现出优异的生物相容性。这些化学修饰的寡核苷酸可以帮助定制预防性抗感染涂层策略,以满足一系列临床挑战,其中金黄色葡萄球菌假体感染的风险从短暂到长期不等。






































 京公网安备 11010802027423号
京公网安备 11010802027423号