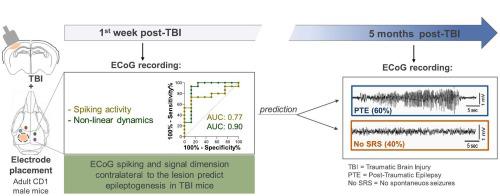
创伤性脑损伤(TBI)和癫痫发作(PTE)之间的潜伏期代表了对抗癫痫发生的机会。抗癫痫发生试验因缺乏敏感的生物标志物而受到阻碍,这些生物标志物无法丰富有 PTE 风险的患者群体。我们的目的是评估特定的 ECoG 信号是否可以在癫痫发病率约 60% 的临床相关小鼠模型中预测 PTE。通过对左侧顶颞叶皮层的受控皮质冲击,在成年 CD1 雄性小鼠中引发 TBI,然后在小鼠病变周围皮层螺钉电极中植入两个类似的电极,并在病变部位对侧的半球中植入两个类似的电极。 TBI 后 1 周内记录急性癫痫发作和尖峰/尖波。这些早期 ECoG 事件根据 PTE 发生率进行分析,PTE 发生率通过测量 TBI 后 5 个月的自发性复发性癫痫发作 (SRS) 进行评估。我们发现,PTE 小鼠和未发生癫痫的小鼠(无 SRS 小鼠)在 TBI 后 3 天内急性癫痫发作的发生率、次数和持续时间相似。使用皮质电极(naïve, n = 5)或使用电极和开颅手术(假手术,n = 5)的对照小鼠表现出急性癫痫发作,但没有发展为癫痫。从受伤后第 2 天起,与对照组( p < 0.05, n = 10)相比,PTE( n = 15)和无 SRS( n = 8)小鼠的病灶周围电极处的每日尖峰/尖波数量也有类似的增加。不同的是,与无 SRS 和对照小鼠相比,PTE 小鼠的两个对侧电极处的每日尖峰/尖波数量逐渐增加。特别是,PTE 小鼠与无 SRS 小鼠相比,尖峰数量更高( p < 0。05)在 TBI 后 6 天和 7 天进行测量,该测量可以高精度预测癫痫的发展(AUC = 0.77, p = 0.03;CI 0.5830–0.9670)。截止值在独立的 TBI 小鼠队列中得到验证( n = 12)。对侧电极处的每日尖峰数量在 PTE 小鼠中显示出昼夜节律分布,而在无 SRS 小鼠中未观察到这一点。对每个电极部位的非线性动力学分析显示了 TBI 后 4 天内的维度变化。该测量在损伤对侧的皮质电极处产生了 PTE 和无 SRS 小鼠之间的最佳区分 ( p < 0.01)。数据显示,在该模型中,病变部位对侧的癫痫样活动对 PTE 具有最高的预测价值,这强化了以下假设:病变核心对侧的半球可能在 TBI 后驱动致癫痫网络。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
ECoG spiking activity and signal dimension are early predictive measures of epileptogenesis in a translational mouse model of traumatic brain injury
The latency between traumatic brain injury (TBI) and the onset of epilepsy (PTE) represents an opportunity for counteracting epileptogenesis. Antiepileptogenesis trials are hampered by the lack of sensitive biomarkers that allow to enrich patient's population at-risk for PTE. We aimed to assess whether specific ECoG signals predict PTE in a clinically relevant mouse model with ∼60% epilepsy incidence. TBI was provoked in adult CD1 male mice by controlled cortical impact on the left parieto-temporal cortex, then mice were implanted with two perilesional cortical screw electrodes and two similar electrodes in the hemisphere contralateral to the lesion site. Acute seizures and spikes/sharp waves were ECoG-recorded during 1 week post-TBI. These early ECoG events were analyzed according to PTE incidence as assessed by measuring spontaneous recurrent seizures (SRS) at 5 months post-TBI. We found that incidence, number and duration of acute seizures during 3 days post-TBI were similar in PTE mice and mice not developing epilepsy (No SRS mice). Control mice with cortical electrodes (naïve, n = 5) or with electrodes and craniotomy (sham, n = 5) exhibited acute seizures but did not develop epilepsy. The daily number of spikes/sharp waves at the perilesional electrodes was increased similarly in PTE (n = 15) and No SRS (n = 8) mice vs controls (p < 0.05, n = 10) from day 2 post-injury. Differently, the daily number of spikes/sharp waves at both contralateral electrodes showed a progressive increase in PTE mice vs No SRS and control mice. In particular, spikes number was higher in PTE vs No SRS mice (p < 0.05) at 6 and 7 days post-TBI, and this measure predicted epilepsy development with high accuracy (AUC = 0.77, p = 0.03; CI 0.5830–0.9670). The cut-off value was validated in an independent cohort of TBI mice (n = 12). The daily spike number at the contralateral electrodes showed a circadian distribution in PTE mice which was not observed in No SRS mice. Analysis of non-linear dynamics at each electrode site showed changes in dimensionality during 4 days post-TBI. This measure yielded the best discrimination between PTE and No SRS mice (p < 0.01) at the cortical electrodes contralateral to injury. Data show that epileptiform activity contralateral to the lesion site has the the highest predictive value for PTE in this model reinforcing the hypothesis that the hemisphere contralateral to the lesion core may drive epileptogenic networks after TBI.





































 京公网安备 11010802027423号
京公网安备 11010802027423号