Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease ( IF 4.2 ) Pub Date : 2023-08-01 , DOI: 10.1016/j.bbadis.2023.166825 Julia Moellmann 1 , Katja Krueger 1 , Dickson W L Wong 2 , Barbara M Klinkhammer 2 , Eva M Buhl 3 , Jonas Dehairs 4 , Johan V Swinnen 4 , Heidi Noels 5 , Joachim Jankowski 5 , Corinna Lebherz 1 , Peter Boor 6 , Nikolaus Marx 1 , Michael Lehrke 1
|
Aim
Chronic kidney disease (CKD) is accompanied by increased cardiovascular risk and heart failure (HF). In rodents, 2,8-dihydroxyadenine (DHA)-induced nephropathy is a frequently used CKD model. Cardiac and kidney tubular cells share high energy demand to guarantee constant contractive force of the heart or reabsorption/secretion of primary filtrated molecules and waste products by the kidney. Here we analyze time-dependent mechanisms of kidney damage and cardiac consequences under consideration of energetic pathways with the focus on mitochondrial function and lipid metabolism in mice.
Methods and results
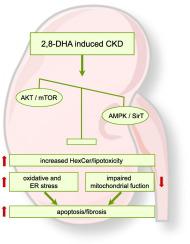
CKD was induced by alternating dietary adenine supplementation (0.2 % or 0.05 % of adenine) in C57BL/6J mice for 9 weeks. Progressive kidney damage led to reduced creatinine clearance, kidney fibrosis and renal inflammation after 3, 6, and 9 weeks. No difference in cardiac function, mitochondrial respiration nor left ventricular fibrosis was observed at any time point. Investigating mechanisms of renal damage, protective SirT3 was decreased in CKD, which contrasted an increase in protein kinase B (AKT) expression, mechanistic target of rapamycin (mTOR) downstream signaling, induction of oxidative and endoplasmic reticulum (ER) stress. This occurred together with impaired renal mitochondrial function and accumulation of hexosylceramides (HexCer) as an established mediator of inflammation and mitochondrial dysfunction in the kidney.
Conclusions
2,8-DHA-induced CKD results in renal activation of the mTOR downstream signaling, endoplasmic reticulum stress, tubular injury, fibrosis, inflammation, oxidative stress and impaired kidney mitochondrial function in conjunction with renal hexosylceramide accumulation in C57BL/6J mice.
中文翻译:

2,8-二羟基腺嘌呤诱导的肾病导致己糖神经酰胺积累,并伴有 mTOR 信号传导增加、保护性 SirT3 表达水平降低和肾线粒体功能受损
目的
慢性肾脏病(CKD)伴随着心血管风险和心力衰竭(HF)的增加。在啮齿类动物中,2,8-二羟基腺嘌呤 (DHA) 诱导的肾病是常用的 CKD 模型。心脏和肾小管细胞共享高能量需求,以保证心脏恒定的收缩力或肾脏对初级过滤分子和废物的重吸收/分泌。在这里,我们在考虑能量途径的情况下分析肾脏损伤和心脏后果的时间依赖性机制,重点关注小鼠的线粒体功能和脂质代谢。
方法和结果
通过在 C57BL/6J 小鼠中交替膳食腺嘌呤补充(0.2% 或 0.05% 腺嘌呤)9 周来诱导 CKD。3、6 和 9 周后,进行性肾脏损伤导致肌酐清除率降低、肾脏纤维化和肾脏炎症。在任何时间点均未观察到心脏功能、线粒体呼吸或左心室纤维化方面的差异。研究肾损伤机制发现,CKD 中保护性 SirT3 减少,而蛋白激酶 B (AKT) 表达、雷帕霉素 (mTOR) 下游信号传导机制靶点、氧化和内质网 (ER) 应激诱导增加形成对比。这种情况与肾脏线粒体功能受损以及己糖神经酰胺 (HexCer) 积累一起发生,己糖神经酰胺是肾脏炎症和线粒体功能障碍的既定介质。
结论
2,8-DHA 诱导的 CKD 导致 mTOR 下游信号传导的肾脏激活、内质网应激、肾小管损伤、纤维化、炎症、氧化应激和肾脏线粒体功能受损,以及 C57BL/6J 小鼠肾己糖神经酰胺的积累。































 京公网安备 11010802027423号
京公网安备 11010802027423号