The Journal of Prevention of Alzheimer's Disease ( IF 8.5 ) Pub Date : 2023-07-12 , DOI: 10.14283/jpad.2023.89
K Önnestam 1 , B Nilsson , M Rother , E Rein-Hedin , J Bylund , P Anderer , M Kemethofer , M M Halldin , J Sandin , M Segerdahl
|
Background
ACD856 is a positive allosteric modulator of tropomyosin receptor kinase (Trk) receptors which has shown to have pro-cognitive and anti-depressant-like effects in various animal models. It is currently in clinical development for the treatment of Alzheimer’s disease and other disorders where cognition is impaired and is also considered for indications such as depression or other neuropsychiatric diseases. ACD856 has a novel mechanism of action modulating the activity of the Trk-receptors, resulting in increased stimulation of the neurotrophin signaling pathways. Previous studies applying single intravenous and oral doses of ACD856 indicate that ACD856 is safe and well-tolerated by healthy volunteer subjects, and that it has suitable safety and pharmacokinetic properties for further clinical development.
Objectives
To investigate the safety and tolerability of 7 days of treatment with multiple ascending oral doses of ACD856 in healthy subjects, and to characterize its pharmacokinetic (PK) properties. In addition, pharmacodynamic effects of ACD856 using quantitative electroencephalography (qEEG) as an indicator for central target engagement were assessed.
Design
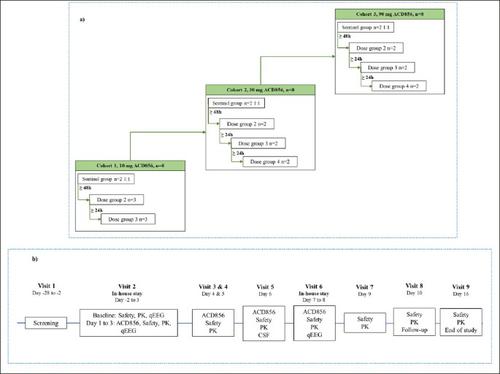
This was a prospective, phase I, double-blind, parallel-group, placebo-controlled, randomized study of the safety, tolerability, PK and pharmacodynamics of multiple ascending oral doses of ACD856 in healthy subjects. ACD856 or placebo were administered in 3 ascending dose cohorts of 8 subjects. Within each cohort, subjects were randomized to receive either ACD856 (n=6) or placebo (n=2).
Setting
The study was conducted at a First-in-Human unit in Sweden.
Participants
Twenty-four healthy male and female subjects.
Intervention
The study medication was administered as an oral solution, with ACD856 or the same contents without the active ingredient (placebo). The dose levels ranged from 10 mg to 90 mg. ACD856 was administered once daily for 7 days, targeting steady state.
Measurements
Safety and tolerability assessments included adverse events, laboratory, vital signs, 12-lead electrocardiogram (ECG), physical examination, assessment of stool frequency and questionnaires to assess symptoms of anxiety, depression, as well as suicidal ideation and behavior. In addition, cardiodynamic ECGs were extracted to evaluate cardiac safety. PK parameters were calculated based on measured concentrations of ACD856 in plasma, urine, and cerebrospinal fluid (CSF) samples. Metabolite profiling, characterization and analysis was performed based on and urine samples. qEEG was recorded for patients in the two highest dose cohorts (30 and 90 mg/day) as a pharmacodynamic assessment to explore central target engagement.
Results
Treatment with ACD856 was well tolerated with no serious adverse events. No treatment emergent or dose related trends were observed for any of the safety assessments. ACD856 was rapidly absorbed and reached maximum plasma exposure at 30 to 45 minutes after administration. Steady state was reached before Day 6, with an elimination half-life at steady state of approximately 20 hours. At steady state, ACD856 exhibited accumulation ratios for Cmax and AUC of approximately 1.6 and 1.9 respectively. The exposure, Cmax and AUC0-24, increased proportionally with the dose. There was no unchanged ACD856 detected in urine. The metabolic pattern in urine and plasma was similar, and in alignment with the metabolites observed in preclinical toxicology studies. The level of ACD856 measured in CSF at steady state increased with dose, indicating Central Nervous System (CNS) exposure at relevant levels for pharmacodynamic effects. ACD856 demonstrated significant dose-dependent treatment-associated changes on qEEG parameters. Specifically, increase of the relative theta power and decrease of the fast alpha and beta power was observed, leading to an acceleration of the delta+theta centroid and an increase in the theta/beta ratio.
Conclusions
ACD856 was well tolerated at the tested dose levels (10–90 mg/daily for 7 days) in healthy subjects. The compound has a robust pharmacokinetic profile, with rapid absorption and dose-dependent exposure. ACD856 was shown to pass the blood-brain-barrier, reach relevant exposure in the CNS and to induce dose-dependent treatment-related changes on qEEG parameters, indicating central target engagement.
中文翻译:

ACD856(一种新型 Trk 受体正变构调节剂)在健康受试者中多次给药后的安全性、耐受性、药代动力学和定量脑电图评估
背景
ACD856 是原肌球蛋白受体激酶 (Trk) 受体的正变构调节剂,已在多种动物模型中显示出具有促进认知和抗抑郁样作用。目前该药物正处于临床开发阶段,用于治疗阿尔茨海默病和其他认知受损的疾病,也被考虑用于治疗抑郁症或其他神经精神疾病等适应症。ACD856 具有调节 Trk 受体活性的新颖作用机制,从而增加对神经营养素信号通路的刺激。先前应用单次静脉注射和口服剂量 ACD856 的研究表明,ACD856 是安全的,并且健康志愿者受试者耐受性良好,并且具有适合进一步临床开发的安全性和药代动力学特性。
目标
研究健康受试者多次递增口服剂量 ACD856 治疗 7 天的安全性和耐受性,并表征其药代动力学 (PK) 特性。此外,使用定量脑电图(qEEG)作为中心靶点参与的指标评估了 ACD856 的药效作用。
设计
这是一项前瞻性、I 期、双盲、平行组、安慰剂对照、随机研究,研究健康受试者多次递增口服剂量 ACD856 的安全性、耐受性、PK 和药效学。ACD856 或安慰剂在 3 个递增剂量组中给予 8 名受试者。在每个队列中,受试者被随机分配接受 ACD856 (n=6) 或安慰剂 (n=2)。
环境
该研究是在瑞典的一个首次人体研究单位进行的。
参加者
二十四名健康男性和女性受试者。
干涉
研究药物以口服溶液形式给药,含有 ACD856 或不含活性成分的相同含量(安慰剂)。剂量水平从10毫克到90毫克不等。ACD856 每天给药一次,持续 7 天,目标是稳定状态。
测量
安全性和耐受性评估包括不良事件、实验室、生命体征、12导联心电图(ECG)、体格检查、大便频率评估以及用于评估焦虑、抑郁症状以及自杀意念和行为的问卷调查。此外,提取心动力心电图以评估心脏安全性。根据血浆、尿液和脑脊液 (CSF) 样本中 ACD856 的测量浓度计算 PK 参数。根据尿液样本进行代谢物分析、表征和分析。记录两个最高剂量组(30 和 90 毫克/天)患者的 qEEG,作为药效学评估,以探索中心靶点参与情况。
结果
ACD856 治疗耐受性良好,未出现严重不良事件。任何安全性评估均未观察到治疗紧急情况或剂量相关趋势。ACD856 被迅速吸收,并在给药后 30 至 45 分钟达到最大血浆暴露量。第 6 天前达到稳定状态,稳定状态下的消除半衰期约为 20 小时。在稳定状态下,ACD856 的 Cmax 和 AUC 累积比率分别约为 1.6 和 1.9。暴露量、Cmax 和 AUC0-24 随剂量成比例增加。尿液中未检测到未变化的 ACD856。尿液和血浆中的代谢模式相似,并且与临床前毒理学研究中观察到的代谢物一致。稳态时脑脊液中测得的 ACD856 水平随剂量增加而增加,表明中枢神经系统 (CNS) 暴露在药效学效应的相关水平。ACD856 在 qEEG 参数上表现出显着的剂量依赖性治疗相关变化。具体来说,观察到相对 theta 功率的增加和快速 alpha 和 beta 功率的减少,导致 delta+theta 质心加速和 theta/beta 比率增加。
结论
在健康受试者中,ACD856 在测试剂量水平(10-90 毫克/每天,持续 7 天)下具有良好的耐受性。该化合物具有强大的药代动力学特征,具有快速吸收和剂量依赖性暴露。ACD856 被证明可以通过血脑屏障,在中枢神经系统中达到相关暴露,并诱导 qEEG 参数的剂量依赖性治疗相关变化,表明中心目标参与。































 京公网安备 11010802027423号
京公网安备 11010802027423号