当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electron-deficient cyclopropenium cations as Lewis acids in FLP chemistry
Chemical Communications ( IF 4.3 ) Pub Date : 2023-08-01 , DOI: 10.1039/d3cc02684a Dipendu Mandal 1, 2 , Zheng-Wang Qu 3 , Stefan Grimme 3 , Douglas W Stephan 1, 2
Chemical Communications ( IF 4.3 ) Pub Date : 2023-08-01 , DOI: 10.1039/d3cc02684a Dipendu Mandal 1, 2 , Zheng-Wang Qu 3 , Stefan Grimme 3 , Douglas W Stephan 1, 2
Affiliation
|
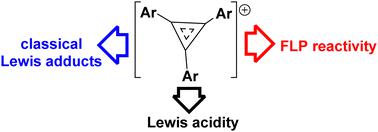
Cyclopropenium cations incorporating electron deficient substituents are Lewis acidic despite the presence of π-electrons. The chloride and electron affinities are examined computationally and experimentally, respectively. These cations form classic Lewis acid-base adducts with PPh3, while sterically demanding phosphines yield frustrated Lewis pairs (FLPs) which participate in FLP additions. Depending on the basicity of the phosphine used, addition to alkynes or alkyne deprotonation is observed. In either case, new C–C bonds are formed, thus extending the utility of the concept of FLP chemistry to these delocalized π-cations.
中文翻译:

FLP 化学中作为路易斯酸的缺电子环丙烯阳离子
尽管存在π电子,包含缺电子取代基的环丙烯阳离子仍呈路易斯酸性。分别通过计算和实验检查氯离子和电子亲和力。这些阳离子与 PPh 3形成经典的路易斯酸碱加合物,而空间要求较高的膦会产生参与 FLP 加成的受挫路易斯对 (FLP)。根据所用膦的碱性,可以观察到与炔烃的加成或炔烃的去质子化。无论哪种情况,都会形成新的 C-C 键,从而将 FLP 化学概念的实用性扩展到这些离域的 π-阳离子。
更新日期:2023-08-01
中文翻译:

FLP 化学中作为路易斯酸的缺电子环丙烯阳离子
尽管存在π电子,包含缺电子取代基的环丙烯阳离子仍呈路易斯酸性。分别通过计算和实验检查氯离子和电子亲和力。这些阳离子与 PPh 3形成经典的路易斯酸碱加合物,而空间要求较高的膦会产生参与 FLP 加成的受挫路易斯对 (FLP)。根据所用膦的碱性,可以观察到与炔烃的加成或炔烃的去质子化。无论哪种情况,都会形成新的 C-C 键,从而将 FLP 化学概念的实用性扩展到这些离域的 π-阳离子。































 京公网安备 11010802027423号
京公网安备 11010802027423号