Cell Chemical Biology ( IF 6.6 ) Pub Date : 2023-07-31 , DOI: 10.1016/j.chembiol.2023.06.026 Elham F Ahanin 1 , Rebecca A Sager 2 , Sarah J Backe 2 , Diana M Dunn 3 , Natela Dushukyan 1 , Adam R Blanden 4 , Nilamber A Mate 5 , Tamie Suzuki 5 , Tyler Anderson 6 , Merin Roy 1 , Jasmeen Oberoi 7 , Chrisostomos Prodromou 8 , Imad Nsouli 2 , Michael Daneshvar 9 , Gennady Bratslavsky 1 , Mark R Woodford 1 , Dimitra Bourboulia 1 , John D Chisholm 5 , Mehdi Mollapour 1
|
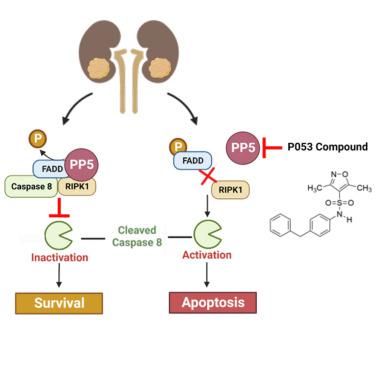
Serine/threonine protein phosphatase-5 (PP5) is involved in tumor progression and survival, making it an attractive therapeutic target. Specific inhibition of protein phosphatases has remained challenging because of their conserved catalytic sites. PP5 contains its regulatory domains within a single polypeptide chain, making it a more desirable target. Here we used an in silico approach to screen and develop a selective inhibitor of PP5. Compound P053 is a competitive inhibitor of PP5 that binds to its catalytic domain and causes apoptosis in renal cancer. We further demonstrated that PP5 interacts with FADD, RIPK1, and caspase 8, components of the extrinsic apoptotic pathway complex II. Specifically, PP5 dephosphorylates and inactivates the death effector protein FADD, preserving complex II integrity and regulating extrinsic apoptosis. Our data suggests that PP5 promotes renal cancer survival by suppressing the extrinsic apoptotic pathway. Pharmacologic inhibition of PP5 activates this pathway, presenting a viable therapeutic strategy for renal cancer.
中文翻译:

蛋白磷酸酶 5 的催化抑制剂通过破坏肾癌中的复合物 II 来激活外源性凋亡途径
丝氨酸/苏氨酸蛋白磷酸酶-5 (PP5) 参与肿瘤进展和生存,使其成为一个有吸引力的治疗靶点。由于其保守的催化位点,蛋白质磷酸酶的特异性抑制仍然具有挑战性。PP5 在单个多肽链中包含其调节结构域,使其成为更理想的靶标。在这里,我们使用计算机模拟方法来筛选和开发 PP5 的选择性抑制剂。化合物 P053 是 PP5 的竞争性抑制剂,可结合其催化结构域并导致肾癌细胞凋亡。我们进一步证明 PP5 与外源性凋亡途径复合物 II 的组分 FADD、RIPK1 和 caspase 8 相互作用。具体来说,PP5 使死亡效应蛋白 FADD 去磷酸化并失活,保持复合物 II 的完整性并调节外源性细胞凋亡。我们的数据表明,PP5 通过抑制外源性凋亡途径促进肾癌存活。PP5 的药物抑制激活了该通路,为肾癌提供了一种可行的治疗策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号