当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh-Catalyzed [4 + 1] Reaction of Cyclopropyl-Capped Dienes (but not Common Dienes) and Carbon Monoxide: Reaction Development and Mechanistic Study
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-31 , DOI: 10.1021/jacs.3c03047 Yusheng Yang 1 , Han-Xiao Li 1 , Tian-Yu Zhu 1 , Zi-You Zhang 1 , Zhi-Xiang Yu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-31 , DOI: 10.1021/jacs.3c03047 Yusheng Yang 1 , Han-Xiao Li 1 , Tian-Yu Zhu 1 , Zi-You Zhang 1 , Zhi-Xiang Yu 1
Affiliation
|
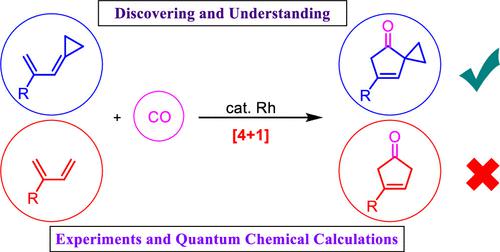
Transition-metal-catalyzed [4 + 1] reaction of dienes and carbon monoxide (CO) is the most straightforward and easily envisioned cyclization for the synthesis of five-membered carbocycles, which are ubiquitously found in natural products and functional molecules. Unfortunately, no test of this reaction was reported, and consequently, chemists do not know whether such kind of reaction works or not. Herein, we report that the [4 + 1] reaction of common dienes and CO cannot work, at least under the catalysis of [Rh(cod)Cl]2. However, using cyclopropyl-capped dienes (also named allylidenecyclopropanes) as substrates, the corresponding [4 + 1] reaction with CO proceeds smoothly in the presence of [Rh(cod)Cl]2. This [4 + 1] reaction, with a broad scope, provides efficient access to five-membered carbocyclic compounds of spiro[2.4]hept-6-en-4-ones. The [4 + 1] cycloadducts can be further transformed into other molecules by using the unique chemistry of cyclopropyl groups present in these molecules. The mechanism of this [4 + 1] reaction has been investigated by quantum chemical calculations, uncovering that cyclopropyl-capped dienes are strained dienes and the oxidative cyclization step in the [4 + 1] catalytic cycle can release this (angular) strain both kinetically and thermodynamically. The strain release in this step then propagates to all followed CO coordination/CO insertion/reductive elimination steps in the [4 + 1] catalytic cycle, helping the realization of this cycloaddition reaction. In contrast, common dienes (including cyclobutyl-capped dienes) do not have such advantages and their [4 + 1] reaction suffers from energy penalty in all steps involved in the [4 + 1] catalytic cycle. The reactivity of ene-allenes for the [4 + 1] reaction with CO is also discussed.
中文翻译:

Rh 催化的环丙基封端二烯(但非普通二烯)与一氧化碳的 [4 + 1] 反应:反应进展和机理研究
过渡金属催化的二烯和一氧化碳 (CO) 的 [4 + 1] 反应是合成五元碳环最直接、最容易设想的环化反应,五元碳环普遍存在于天然产物和功能分子中。不幸的是,没有报道这种反应的测试,因此化学家不知道这种反应是否有效。在此,我们报道了普通二烯和CO的[4+1]反应不能进行,至少在[Rh(cod)Cl] 2的催化下是这样。然而,使用环丙基封端的二烯(也称为烯丙基环丙烷)作为底物,相应的与CO的[4+1]反应在[Rh(cod)Cl] 2存在下顺利进行。这种[4 + 1]反应具有广泛的范围,可以有效地获得螺[2.4]hept-6-en-4-ones的五元碳环化合物。通过使用这些分子中存在的环丙基的独特化学性质,[4 + 1]环加合物可以进一步转化为其他分子。通过量子化学计算研究了这种[4 + 1]反应的机理,发现环丙基封端的二烯是应变二烯,并且[4 + 1]催化循环中的氧化环化步骤可以在动力学上释放这种(角)应变和热力学上。该步骤中的应变释放随后传播到[4+1]催化循环中所有后续的CO配位/CO插入/还原消除步骤,有助于该环加成反应的实现。相比之下,普通二烯(包括环丁基封端二烯)不具有这些优点,并且它们的[4+1]反应在[4+1]催化循环涉及的所有步骤中都遭受能量损失。还讨论了烯-丙二烯与 CO 的 [4 + 1] 反应的反应性。
更新日期:2023-07-31
中文翻译:

Rh 催化的环丙基封端二烯(但非普通二烯)与一氧化碳的 [4 + 1] 反应:反应进展和机理研究
过渡金属催化的二烯和一氧化碳 (CO) 的 [4 + 1] 反应是合成五元碳环最直接、最容易设想的环化反应,五元碳环普遍存在于天然产物和功能分子中。不幸的是,没有报道这种反应的测试,因此化学家不知道这种反应是否有效。在此,我们报道了普通二烯和CO的[4+1]反应不能进行,至少在[Rh(cod)Cl] 2的催化下是这样。然而,使用环丙基封端的二烯(也称为烯丙基环丙烷)作为底物,相应的与CO的[4+1]反应在[Rh(cod)Cl] 2存在下顺利进行。这种[4 + 1]反应具有广泛的范围,可以有效地获得螺[2.4]hept-6-en-4-ones的五元碳环化合物。通过使用这些分子中存在的环丙基的独特化学性质,[4 + 1]环加合物可以进一步转化为其他分子。通过量子化学计算研究了这种[4 + 1]反应的机理,发现环丙基封端的二烯是应变二烯,并且[4 + 1]催化循环中的氧化环化步骤可以在动力学上释放这种(角)应变和热力学上。该步骤中的应变释放随后传播到[4+1]催化循环中所有后续的CO配位/CO插入/还原消除步骤,有助于该环加成反应的实现。相比之下,普通二烯(包括环丁基封端二烯)不具有这些优点,并且它们的[4+1]反应在[4+1]催化循环涉及的所有步骤中都遭受能量损失。还讨论了烯-丙二烯与 CO 的 [4 + 1] 反应的反应性。













































 京公网安备 11010802027423号
京公网安备 11010802027423号