Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction mechanism and kinetics of the two-component flavoprotein dimethyl sulfone monooxygenase system: Using hydrogen peroxide for monooxygenation and substrate cleavage
The FEBS Journal ( IF 5.5 ) Pub Date : 2023-07-31 , DOI: 10.1111/febs.16916 Montisa Mangkalee 1, 2 , Worrapoj Oonanant 3 , Chanat Aonbangkhen 1, 4 , Panu Pimviriyakul 5 , Ruchanok Tinikul 6 , Pimchai Chaiyen 7 , Numpon Insin 1, 2 , Jeerus Sucharitakul 8, 9
The FEBS Journal ( IF 5.5 ) Pub Date : 2023-07-31 , DOI: 10.1111/febs.16916 Montisa Mangkalee 1, 2 , Worrapoj Oonanant 3 , Chanat Aonbangkhen 1, 4 , Panu Pimviriyakul 5 , Ruchanok Tinikul 6 , Pimchai Chaiyen 7 , Numpon Insin 1, 2 , Jeerus Sucharitakul 8, 9
Affiliation
|
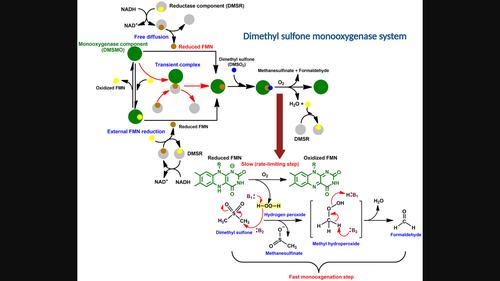
The dimethyl sulfone monooxygenase system is a two-component flavoprotein, catalyzing the monooxygenation of dimethyl sulfone (DMSO2) by oxidative cleavage producing methanesulfinate and formaldehyde. The reductase component (DMSR) is a flavoprotein with FMN as a cofactor, catalyzing flavin reduction using NADH. The monooxygenase (DMSMO) uses reduced flavin from the reductase and oxygen for substrate monooxygenation. DMSMO can bind to FMN and FMNH− with a Kd of 17.4 ± 0.9 μm and 4.08 ± 0.8 μm, respectively. The binding of FMN to DMSMO is required prior to binding DMSO2. This also applies to the fast binding of reduced FMN to DMSMO followed by DMSO2. Substituting reduced DMSR with FMNH− demonstrated the same oxidation kinetics, indicating that FMNH− from DMSR was transferred to DMSMO. The oxidation of FMNH−:DMSMO, with and without DMSO2 did not generate any flavin adducts for monooxygenation. Therefore, H2O2 is likely to be the reactive agent to attack the substrate. The H2O2 assay results demonstrated production of H2O2 from the oxidation of FMNH−:DMSMO, whereas H2O2 was not detected in the presence of DMSO2, confirming H2O2 utilization. The rate constant for methanesulfinate formation determined from rapid quenched flow and the rate constant for flavin oxidation were similar, indicating that H2O2 rapidly reacts with DMSO2, with flavin oxidation as the rate-limiting step. This is the first report of the kinetic mechanisms of both components using rapid kinetics and of a method for methanesulfinate detection using LC–MS.
中文翻译:

双组分黄素蛋白二甲基砜单加氧酶系统的反应机制和动力学:使用过氧化氢进行单加氧和底物裂解
二甲基砜单加氧酶系统是一种双组分黄素蛋白,通过氧化裂解催化二甲基砜(DMSO 2 )的单加氧,产生甲亚磺酸盐和甲醛。还原酶成分 (DMSR) 是一种黄素蛋白,以 FMN 作为辅助因子,利用 NADH 催化黄素还原。单加氧酶 (DMSMO) 使用还原酶中的还原黄素和氧气进行底物单加氧。DMSMO 可以与 FMN 和 FMNH −结合,K d分别为17.4 ± 0.9 μm和4.08 ± 0.8 μm。在结合 DMSO 2之前,需要将 FMN 与 DMSMO 结合。这也适用于还原的 FMN 与 DMSMO 的快速结合,然后是 DMSO 2。用 FMNH -替代还原的 DMSR表现出相同的氧化动力学,表明 FMNH -从 DMSR 转移到了 DMSMO。FMNH - :DMSMO的氧化,无论有无 DMSO 2 ,均不会产生任何用于单氧化的黄素加合物。因此,H 2 O 2很可能是攻击基材的反应剂。H 2 O 2测定结果表明, FMNH - :DMSMO的氧化产生了 H 2 O 2,而在 DMSO 2存在的情况下没有检测到H 2 O 2,这证实了 H 2 O 2的利用。由快速骤冷流确定的甲亚磺酸盐形成的速率常数与黄素氧化的速率常数相似,表明H 2 O 2与DMSO 2快速反应,以黄素氧化作为限速步骤。这是使用快速动力学研究两种组分的动力学机制以及使用 LC-MS 检测甲亚磺酸盐的方法的首次报告。
更新日期:2023-07-31
中文翻译:

双组分黄素蛋白二甲基砜单加氧酶系统的反应机制和动力学:使用过氧化氢进行单加氧和底物裂解
二甲基砜单加氧酶系统是一种双组分黄素蛋白,通过氧化裂解催化二甲基砜(DMSO 2 )的单加氧,产生甲亚磺酸盐和甲醛。还原酶成分 (DMSR) 是一种黄素蛋白,以 FMN 作为辅助因子,利用 NADH 催化黄素还原。单加氧酶 (DMSMO) 使用还原酶中的还原黄素和氧气进行底物单加氧。DMSMO 可以与 FMN 和 FMNH −结合,K d分别为17.4 ± 0.9 μm和4.08 ± 0.8 μm。在结合 DMSO 2之前,需要将 FMN 与 DMSMO 结合。这也适用于还原的 FMN 与 DMSMO 的快速结合,然后是 DMSO 2。用 FMNH -替代还原的 DMSR表现出相同的氧化动力学,表明 FMNH -从 DMSR 转移到了 DMSMO。FMNH - :DMSMO的氧化,无论有无 DMSO 2 ,均不会产生任何用于单氧化的黄素加合物。因此,H 2 O 2很可能是攻击基材的反应剂。H 2 O 2测定结果表明, FMNH - :DMSMO的氧化产生了 H 2 O 2,而在 DMSO 2存在的情况下没有检测到H 2 O 2,这证实了 H 2 O 2的利用。由快速骤冷流确定的甲亚磺酸盐形成的速率常数与黄素氧化的速率常数相似,表明H 2 O 2与DMSO 2快速反应,以黄素氧化作为限速步骤。这是使用快速动力学研究两种组分的动力学机制以及使用 LC-MS 检测甲亚磺酸盐的方法的首次报告。































 京公网安备 11010802027423号
京公网安备 11010802027423号