当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How do donor and acceptor substituents change the photophysical and photochemical behavior of dithienylethenes? The search for a water-soluble visible-light photoswitch
Chemical Science ( IF 7.6 ) Pub Date : 2023-07-31 , DOI: 10.1039/d3sc01458d Sili Qiu 1 , Andrew T Frawley 1 , Kathryn G Leslie 1 , Harry L Anderson 1
Chemical Science ( IF 7.6 ) Pub Date : 2023-07-31 , DOI: 10.1039/d3sc01458d Sili Qiu 1 , Andrew T Frawley 1 , Kathryn G Leslie 1 , Harry L Anderson 1
Affiliation
|
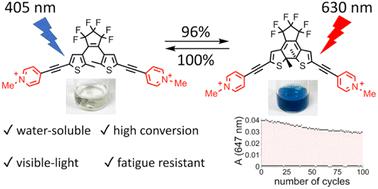
Dithienylethenes are a type of diarylethene and they constitute one of the most widely studied classes of photoswitch, yet there have been no systematic studies of how electron-donor or -acceptor substituents affect their properties. Here we report eight dithienylethenes bearing push–push, pull–pull and push–pull substitution patterns with different lengths of conjugation in the backbone and investigate their photophysical and photochemical properties. Donor–acceptor interactions in the closed forms of push–pull dithienylethenes shift their absorption spectra into the near-infrared region (λmax ≈ 800 nm). The push–pull systems also exhibit low quantum yields for photochemical electrocyclization, and computational studies indicate that this can be attributed to stabilization of the parallel, rather than anti-parallel, conformations. The pull–pull systems have the highest quantum yields for switching in both directions, ring-closure and ring-opening. The chloride salt of a pull–pull DTE, with alkynes on both arms, is the first water-soluble dithienylethene that can achieve >95% photostationary state distribution in both directions with visible light. It has excellent fatigue resistance: in aqueous solution on irradiation at 365 nm, the photochemical quantum yields for switching and decomposition are 0.15 and 2.6 × 10−5 respectively, i.e. decomposition is more than 5000 times slower than photoswitching. These properties make it a promising candidate for biological applications such as super-resolution microscopy and photopharmacology.
中文翻译:

供体和受体取代基如何改变二噻吩乙烯的光物理和光化学行为?寻找水溶性可见光光电开关
二噻吩乙烯是二芳基乙烯的一种,它们构成了研究最广泛的光开关类别之一,但尚未对电子供体或受体取代基如何影响其特性进行系统研究。在这里,我们报道了主链中具有不同共轭长度的八种具有推-推、拉-拉和推-拉取代模式的二噻吩乙烯,并研究了它们的光物理和光化学性质。推拉二噻吩乙烯闭合形式中的供体-受体相互作用将其吸收光谱转移到近红外区域(λ max ≈ 800 nm)。推拉系统还表现出光化学电环化的低量子产率,计算研究表明这可以归因于平行构象的稳定,而不是反平行构象的稳定。拉-拉系统在闭环和开环两个方向的切换方面具有最高的量子产率。双臂上带有炔烃的拉-拉 DTE 的氯化物盐是第一种水溶性二噻吩乙烯,可以在可见光的两个方向上实现 >95% 的光稳态分布。其具有优异的抗疲劳性:在水溶液中,在365 nm照射下,开关和分解的光化学量子产率分别为0.15和2.6×10 -5,即分解比光开关慢5000倍以上。这些特性使其成为超分辨率显微镜和光药理学等生物应用的有前途的候选者。
更新日期:2023-07-31
中文翻译:

供体和受体取代基如何改变二噻吩乙烯的光物理和光化学行为?寻找水溶性可见光光电开关
二噻吩乙烯是二芳基乙烯的一种,它们构成了研究最广泛的光开关类别之一,但尚未对电子供体或受体取代基如何影响其特性进行系统研究。在这里,我们报道了主链中具有不同共轭长度的八种具有推-推、拉-拉和推-拉取代模式的二噻吩乙烯,并研究了它们的光物理和光化学性质。推拉二噻吩乙烯闭合形式中的供体-受体相互作用将其吸收光谱转移到近红外区域(λ max ≈ 800 nm)。推拉系统还表现出光化学电环化的低量子产率,计算研究表明这可以归因于平行构象的稳定,而不是反平行构象的稳定。拉-拉系统在闭环和开环两个方向的切换方面具有最高的量子产率。双臂上带有炔烃的拉-拉 DTE 的氯化物盐是第一种水溶性二噻吩乙烯,可以在可见光的两个方向上实现 >95% 的光稳态分布。其具有优异的抗疲劳性:在水溶液中,在365 nm照射下,开关和分解的光化学量子产率分别为0.15和2.6×10 -5,即分解比光开关慢5000倍以上。这些特性使其成为超分辨率显微镜和光药理学等生物应用的有前途的候选者。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号