Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2023-07-28 , DOI: 10.1016/j.bioorg.2023.106748
Jiuzhong Huang 1 , Yi Wu 2 , Zhihao Hu 1 , Shihong Han 1 , Lanlan Rong 1 , Xin Xie 1 , Weiming Chen 1 , Xiaopeng Peng 1
|
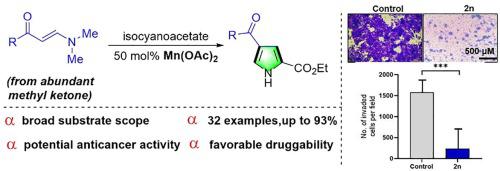
The practical and facile Mn(OAc)2-promoted [3+2] cycloaddition reaction of enaminones with isocyanoacetate was developed, that delivered a diversity of 3-aroyl pyrrole-2-carboxylic esters with broad substrates scope. The most of the newly synthesized compounds exhibit moderate antiproliferative activity against four cancer cells. Notably, compound 2n demonstrate the most potent activity with average IC50 values of 5.61 μM against four distinct cancer cell lines. Moreover, 2n exhibit favorable anti-migration activity and drug-like properties. The further investigation suggests that compound 2n possesses the ability to inhibit ERK5 activity and exhibits effective binding with the ERK5 protein, making it a promising candidate as a lead compound for a new class of ERK5 inhibitors discovery.
中文翻译:

Mn(OAc)2-促进烯胺酮与异氰乙酸酯的[3+2]环化:快速获得具有有效抗癌活性的吡咯-2-羧酸酯衍生物
开发了实用且简便的Mn(OAc) 2促进的烯胺酮与异氰乙酸酯的[3+2]环加成反应,产生了多种具有广泛底物范围的3-芳酰基吡咯-2-羧酸酯。大多数新合成的化合物对四种癌细胞表现出中等的抗增殖活性。值得注意的是,化合物2n对四种不同的癌细胞系表现出最有效的活性,平均 IC 50值为 5.61 μM。此外,2n表现出良好的抗迁移活性和药物样特性。进一步的研究表明,化合物2n具有抑制ERK5活性的能力,并表现出与ERK5蛋白的有效结合,使其成为一类新型ERK5抑制剂发现的先导化合物的有希望的候选者。































 京公网安备 11010802027423号
京公网安备 11010802027423号