Chem Catalysis ( IF 11.5 ) Pub Date : 2023-07-28 , DOI: 10.1016/j.checat.2023.100705 Bo Huang , Yuhan Liu , Hirokazu Kobayashi , Zhe Tan , Tomokazu Yamamoto , Takaaki Toriyama , Syo Matsumura , Shogo Kawaguchi , Yoshiki Kubota , Hong Zheng , Hiroshi Kitagawa
|
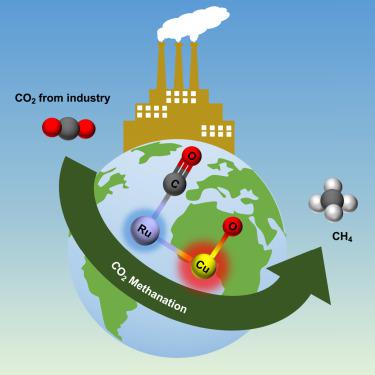
CO2 methanation is a promising large-scale carbon neutralization process for industrial exhaust gases. However, the moderate activities of catalysts to date have always limited large-scale practical applications of CO2 methanation. Solid solutions with abundant active sites and adjustable electronic states are good candidates for achieving higher activities. Here, we developed a carbon neutralization platform system and synthesized CuxRu1-x solid solutions for CO2 methanation by coreduction methodology. Among them, Cu0.05Ru0.95 showed the best CO2 methanation activity, outperforming the best monometallic catalyst Ru. The origin of excellent activity was attributed to the rapid CO2 asymmetric dissociation on Cu-Ru dual-atom sites. Furthermore, anomalous electron transfer from Ru to Cu was found, resulting in high electron density in Cu-atom sites, which mainly induced the rapid CO2 dissociation for CuxRu1-x solid solutions. In addition, the Cu0.7Ru0.3 solid solution had high intrinsic activity for CO2 hydrogenation and 100% CO selectivity.
中文翻译:

用于生产 CH4 或 CO 的碳中和的 CuxRu1-x 催化剂
CO 2甲烷化是一种很有前景的大规模工业废气碳中和工艺。然而,迄今为止催化剂的活性较差一直限制着CO 2甲烷化的大规模实际应用。具有丰富活性位点和可调节电子态的固体溶液是实现更高活性的良好候选者。在这里,我们开发了碳中和平台系统,并通过共还原方法合成了用于CO 2甲烷化的Cu x Ru 1-x固溶体。其中Cu 0.05 Ru 0.95 CO 2表现最好甲烷化活性,优于最好的单金属催化剂Ru。优异活性的起源归因于Cu-Ru双原子位点上的快速CO 2不对称解离。此外,还发现了从Ru到Cu的反常电子转移,导致Cu原子位点上的电子密度较高,这主要诱导了Cu x Ru 1-x固溶体的快速CO 2解离。此外,Cu 0.7 Ru 0.3固溶体具有较高的CO 2加氢本征活性和100%的CO选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号