Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2023-07-28 , DOI: 10.1016/j.cej.2023.145012 Jia Huang , Yaobin Ding , Yang Zong , Deli Wu , Jizhou Jiang , Xingmao Jiang
|
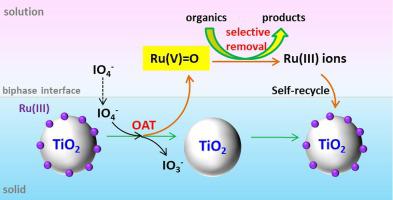
Recently, metal catalysts have received much attention in periodate (PI) activation for production of reactive oxygen species to efficiently degrade organic contaminants, yet activation efficiency is cumbered with their sluggish catalytic cycle. In the study, TiO2 supported Ru(III) catalysts (Ru(III)/TiO2, 0.45 wt% as Ru) were used to catalyze PI activation for oxidation of aqueous organic pollutants. Ru(III)/TiO2 exhibited comparable performance with homogeneous Ru(III) for phenol degradation via PI activation at initial pH = 4, 7 and 10. Moreover, the Ru(III)/TiO2-PI system showed high selectivity for degradation of electron-rich organic contaminants. In comparison, benzoic acid and ibuprofen were hardly oxidized in the system. Scavenging tests, H218O-isotope labeled methyl phenyl sulfoxide (PMSO) probe experiments, X-ray absorption near-edge structure spectra and Electron Spin Resonance analysis confirm formation of Ru(V) = O species as dominant oxidant in the process with little involvement of IO3 ,
,  OH and O2
OH and O2 −. The formed Ru(V) = O species were further confirmed to be water-soluble via analysis of Ru distribution between aqueous solution and TiO2 surface. They can be reduced to Ru(III) species by reacting with organics, realizing the catalytic cycle of Ru(III) → Ru(V) = O → Ru(III) and self-recycle of Ru(III) onto TiO2 surface. In addition, PI was decomposed in stoichiometry into IO3– without generation of undesired iodine species (i.e., HOI and I2). This study advances understandings of PI activation mechanism by heterogeneous Ru(III) catalysts, and also provides a promising oxidation technology for wastewater purification.
−. The formed Ru(V) = O species were further confirmed to be water-soluble via analysis of Ru distribution between aqueous solution and TiO2 surface. They can be reduced to Ru(III) species by reacting with organics, realizing the catalytic cycle of Ru(III) → Ru(V) = O → Ru(III) and self-recycle of Ru(III) onto TiO2 surface. In addition, PI was decomposed in stoichiometry into IO3– without generation of undesired iodine species (i.e., HOI and I2). This study advances understandings of PI activation mechanism by heterogeneous Ru(III) catalysts, and also provides a promising oxidation technology for wastewater purification.
中文翻译:

自循环 Ru(III)/TiO2 活化高碘酸盐选择性氧化水性有机污染物:均相 Ru(V)=O 物种的重要作用
近年来,金属催化剂在高碘酸盐(PI)活化中产生活性氧以有效降解有机污染物而受到广泛关注,但其缓慢的催化循环阻碍了活化效率。在该研究中,TiO 2负载Ru(III)催化剂(Ru(III)/TiO 2 , 0.45 wt% Ru)被用于催化PI活化以氧化水性有机污染物。Ru(III)/TiO 2在初始 pH = 4、7 和 10 下通过 PI 活化降解苯酚时表现出与均质 Ru(III) 相当的性能。此外,Ru(III)/TiO 2-PI系统对富电子有机污染物的降解表现出高选择性。相比之下,苯甲酸和布洛芬在体系中几乎不被氧化。清除测试、H 2 18 O-同位素标记的甲基苯基亚砜 (PMSO) 探针实验、X 射线吸收近边结构 光谱和电子自旋共振分析证实,Ru(V) = O 物种在该过程中作为主要氧化剂形成。 IO 3 、
、 OH和O 2
OH和O 2  -的参与很少。通过分析水溶液和TiO 2之间的Ru分布,进一步证实所形成的Ru(V) = O物种是水溶性的 表面。它们可以通过与有机物反应被还原为Ru(III)物种,实现Ru(III)→Ru(V)=O→Ru(III)的催化循环以及Ru(III)在TiO 2 表面上的自循环。此外,PI 按化学计量分解为 IO 3 – 不会生成不需要的碘物质(即 HOI 和 I 2)。这项研究加深了对非均相Ru(III)催化剂的PI活化机制的理解,并为废水净化提供了一种有前景的氧化技术。
-的参与很少。通过分析水溶液和TiO 2之间的Ru分布,进一步证实所形成的Ru(V) = O物种是水溶性的 表面。它们可以通过与有机物反应被还原为Ru(III)物种,实现Ru(III)→Ru(V)=O→Ru(III)的催化循环以及Ru(III)在TiO 2 表面上的自循环。此外,PI 按化学计量分解为 IO 3 – 不会生成不需要的碘物质(即 HOI 和 I 2)。这项研究加深了对非均相Ru(III)催化剂的PI活化机制的理解,并为废水净化提供了一种有前景的氧化技术。































 京公网安备 11010802027423号
京公网安备 11010802027423号