Cell Calcium ( IF 4.3 ) Pub Date : 2023-07-29 , DOI: 10.1016/j.ceca.2023.102784 Marisa Brini 1 , Tito Calì 2
|
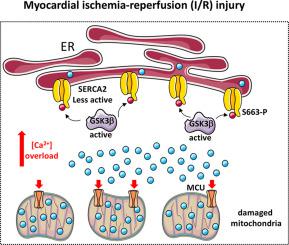
Gonnot et al. [1] thoroughly investigated the regulatory role of glycogen synthase kinase 3 beta (GSK3β) in modulating cardiac isoform 2 of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA2) activity. They have found that in ischemic hearts of patients and mouse-GSK3β -mediated SERCA2 phosphorylation at serine 663 dampens the SERCA2 pump activity and induces Ca2+ overload which sensitizes towards myocardial ischemia-reperfusion (I/R) injury. The inhibition of serine 663 phosphorylation significantly increases SERCA2 activity and, by preventing cytosolic and mitochondrial Ca2+ overload, reduces cell death during reperfusion. Augmented SERCA2 activity also substantially improves excitation-contraction coupling in cardiomyocytes upon recovery from reperfusion injury. This study provides valuable insights into pathophysiological relevance of GSK3β -mediated SERCA2 phosphorylation in the context of heart diseases and paves the way for designing novel clinical therapeutic approaches to alleviate post infartion heart failure.
中文翻译:

SERCA2 磷酸化是疾病的核心
贡诺特等人。 [1] 深入研究了糖原合成酶激酶 3 beta (GSK3β) 在调节肌浆/内质网 Ca 2+ ATP 酶 (SERCA2) 活性的心脏亚型 2 中的调节作用。他们发现,在患者缺血心脏中,小鼠 GSK3β 介导的 SERCA2 丝氨酸 663 磷酸化会抑制 SERCA2 泵活性并诱导 Ca 2+ 超载,从而对心肌缺血再灌注 (I/R) 损伤敏感。丝氨酸 663 磷酸化的抑制显着增加 SERCA2 活性,并通过防止胞质和线粒体 Ca 2+ 超载,减少再灌注期间的细胞死亡。增强的 SERCA2 活性还可以显着改善再灌注损伤恢复后心肌细胞的兴奋-收缩耦合。这项研究为 GSK3β 介导的 SERCA2 磷酸化在心脏病中的病理生理学相关性提供了有价值的见解,并为设计新的临床治疗方法来缓解梗死后心力衰竭铺平了道路。































 京公网安备 11010802027423号
京公网安备 11010802027423号