当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2H-Phosphindole-Enabled Dearomatization and [4+2] Cycloaddition of (Hetero)Arenes
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2023-07-28 , DOI: 10.1002/chem.202301898 Haotian Luo 1 , Junjian Wang 1 , Rongqiang Tian 1 , Zheng Duan 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2023-07-28 , DOI: 10.1002/chem.202301898 Haotian Luo 1 , Junjian Wang 1 , Rongqiang Tian 1 , Zheng Duan 1
Affiliation
|
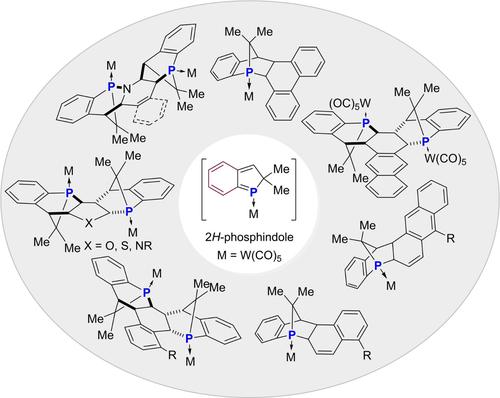
Due to their unique electronic properties, the low-coordinate heavier main-group element species display high reactivity toward inert small molecules. Transient 2H-phosphinidole working as reactive phosphadiene undergoes phospha-Diels-Alder reaction with a wide range of non-activated aromatic carbocycles and heterocycles, providing simple but straightforward access to a range of polycyclic fused rings feature with bridgehead phosphorus.
中文翻译:

(杂)芳烃的 2H-膦吲哚脱芳构化和 [4+2] 环加成
由于其独特的电子特性,低配位较重主族元素物种对惰性小分子表现出高反应性。作为反应性磷二烯的瞬态 2 H-次膦酰亚胺会与各种非活化芳香碳环和杂环发生磷-狄尔斯-阿尔德反应,从而简单而直接地获得一系列具有桥头磷的多环稠合环。
更新日期:2023-07-28
中文翻译:

(杂)芳烃的 2H-膦吲哚脱芳构化和 [4+2] 环加成
由于其独特的电子特性,低配位较重主族元素物种对惰性小分子表现出高反应性。作为反应性磷二烯的瞬态 2 H-次膦酰亚胺会与各种非活化芳香碳环和杂环发生磷-狄尔斯-阿尔德反应,从而简单而直接地获得一系列具有桥头磷的多环稠合环。















































 京公网安备 11010802027423号
京公网安备 11010802027423号