当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computation of Electrical Conductivities of Aqueous Electrolyte Solutions: Two Surfaces, One Property
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2023-07-28 , DOI: 10.1021/acs.jctc.3c00562 Samuel Blazquez 1 , Jose L F Abascal 1 , Jelle Lagerweij 2 , Parsa Habibi 2, 3 , Poulumi Dey 3 , Thijs J H Vlugt 2 , Othonas A Moultos 2 , Carlos Vega 1
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2023-07-28 , DOI: 10.1021/acs.jctc.3c00562 Samuel Blazquez 1 , Jose L F Abascal 1 , Jelle Lagerweij 2 , Parsa Habibi 2, 3 , Poulumi Dey 3 , Thijs J H Vlugt 2 , Othonas A Moultos 2 , Carlos Vega 1
Affiliation
|
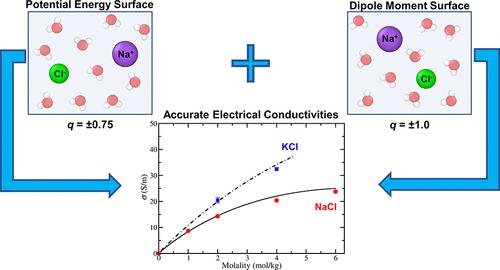
In this work, we computed electrical conductivities under ambient conditions of aqueous NaCl and KCl solutions by using the Einstein–Helfand equation. Common force fields (charge q = ±1 e) do not reproduce the experimental values of electrical conductivities, viscosities, and diffusion coefficients. Recently, we proposed the idea of using different charges to describe the potential energy surface (PES) and the dipole moment surface (DMS). In this work, we implement this concept. The equilibrium trajectories required to evaluate electrical conductivities (within linear response theory) were obtained by using scaled charges (with the value q = ±0.75 e) to describe the PES. The potential parameters were those of the Madrid-Transport force field, which accurately describe viscosities and diffusion coefficients of these ionic solutions. However, integer charges were used to compute the conductivities (thus describing the DMS). The basic idea is that although the scaled charge describes the ion–water interaction better, the integer charge reflects the value of the charge that is transported due to the electric field. The agreement obtained with experiments is excellent, as for the first time electrical conductivities (and the other transport properties) of NaCl and KCl electrolyte solutions are described with high accuracy for the whole concentration range up to their solubility limit. Finally, we propose an easy way to obtain a rough estimate of the actual electrical conductivity of the potential model under consideration using the approximate Nernst–Einstein equation, which neglects correlations between different ions.
中文翻译:

电解质水溶液电导率的计算:两个表面,一种性质
在这项工作中,我们使用爱因斯坦-赫尔芬德方程计算了 NaCl 和 KCl 水溶液在环境条件下的电导率。常见的力场(电荷q = ±1 e )不能再现电导率、粘度和扩散系数的实验值。最近,我们提出了使用不同电荷来描述势能面(PES)和偶极矩面(DMS)的想法。在这项工作中,我们实践了这个概念。评估电导率(在线性响应理论内)所需的平衡轨迹是通过使用缩放电荷(值q = ±0.75 e )来描述 PES 获得的。潜在参数是马德里传输力场的参数,它准确地描述了这些离子溶液的粘度和扩散系数。然而,整数电荷用于计算电导率(从而描述 DMS)。基本思想是,虽然按比例缩放的电荷更好地描述了离子-水相互作用,但整数电荷反映了由于电场而传输的电荷值。实验获得的一致性非常好,因为首次在整个浓度范围内(直至其溶解度极限)高精度地描述了 NaCl 和 KCl 电解质溶液的电导率(以及其他传输特性)。最后,我们提出了一种简单的方法,使用近似能斯特-爱因斯坦方程来粗略估计所考虑的电势模型的实际电导率,该方程忽略了不同离子之间的相关性。
更新日期:2023-07-28
中文翻译:

电解质水溶液电导率的计算:两个表面,一种性质
在这项工作中,我们使用爱因斯坦-赫尔芬德方程计算了 NaCl 和 KCl 水溶液在环境条件下的电导率。常见的力场(电荷q = ±1 e )不能再现电导率、粘度和扩散系数的实验值。最近,我们提出了使用不同电荷来描述势能面(PES)和偶极矩面(DMS)的想法。在这项工作中,我们实践了这个概念。评估电导率(在线性响应理论内)所需的平衡轨迹是通过使用缩放电荷(值q = ±0.75 e )来描述 PES 获得的。潜在参数是马德里传输力场的参数,它准确地描述了这些离子溶液的粘度和扩散系数。然而,整数电荷用于计算电导率(从而描述 DMS)。基本思想是,虽然按比例缩放的电荷更好地描述了离子-水相互作用,但整数电荷反映了由于电场而传输的电荷值。实验获得的一致性非常好,因为首次在整个浓度范围内(直至其溶解度极限)高精度地描述了 NaCl 和 KCl 电解质溶液的电导率(以及其他传输特性)。最后,我们提出了一种简单的方法,使用近似能斯特-爱因斯坦方程来粗略估计所考虑的电势模型的实际电导率,该方程忽略了不同离子之间的相关性。































 京公网安备 11010802027423号
京公网安备 11010802027423号