当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of 1-Aminoindanes via [3 + 2] Annulation of Aldimines with Alkenes by Scandium-Catalyzed C–H Activation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-28 , DOI: 10.1021/jacs.3c06482
Aniket Mishra 1 , Xuefeng Cong 1 , Masayoshi Nishiura 1, 2 , Zhaomin Hou 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-07-28 , DOI: 10.1021/jacs.3c06482
Aniket Mishra 1 , Xuefeng Cong 1 , Masayoshi Nishiura 1, 2 , Zhaomin Hou 1, 2
Affiliation
|
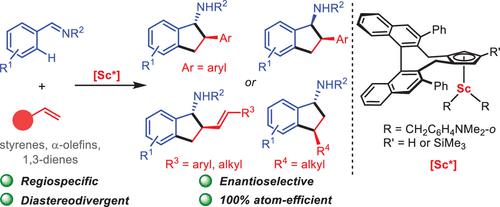
Multisubstituted chiral 1-aminoindanes are important components in many pharmaceuticals and bioactive molecules. Therefore, the development of efficient and selective methods for the synthesis of chiral 1-aminoindanes is of great interest and importance. In principle, the asymmetric [3 + 2] annulation of aldimines with alkenes through C–H activation is the most atom-efficient and straightforward route for the construction of chiral 1-aminoindanes, but such a transformation has remained undeveloped to date probably due to the lack of suitable catalysts. Herein, we report for the first time the enantioselective [3 + 2] annulation of a wide range of aromatic aldimines and alkenes via ortho-C(sp2)–H activation by chiral half-sandwich scandium catalysts, which provides a straightforward route for the synthesis of multisubstituted chiral 1-aminoindanes. This protocol features 100% atom-efficiency, broad functional group compatibility, and high regio-, diastereo-, and enantioselectivity (up to >19:1 dr and 99:1 er). Remarkably, by fine-tuning the sterics of the chiral ligand around the catalyst metal center, the diastereodivergent asymmetric [3 + 2] annulation of aldimines and styrenes has been achieved with a high level of diastereo- and enantioselectivity, offering an efficient method for the synthesis of both the trans and cis diastereomers of a novel class of chiral 1-aminoindane derivatives containing two contiguous stereocenters from the same set of starting materials. Moreover, the asymmetric [3 + 2] annulation of aldimines with aliphatic α-olefins, norbornene, and 1,3-dienes has also been achieved.
中文翻译:

钪催化 C-H 活化通过醛亚胺与烯烃的 [3 + 2] 环化反应对映选择性合成 1-氨基茚满
多取代手性 1-氨基茚满是许多药物和生物活性分子的重要成分。因此,开发有效且选择性的手性1-氨基茚满合成方法具有重大意义和重要性。原则上,醛亚胺与烯烃通过 C-H 活化进行不对称 [3 + 2] 环化是构建手性 1-氨基茚满的原子效率最高且最直接的途径,但迄今为止这种转化仍未得到开发,可能是由于缺乏合适的催化剂。在此,我们首次报道了通过手性半夹心钪催化剂的邻-C(sp 2 )–H活化对多种芳香醛亚胺和烯烃进行对映选择性[3 + 2]环化,这为多取代手性1-氨基茚满的合成。该方案具有 100% 原子效率、广泛的官能团兼容性以及高区域选择性、非对映选择性和对映选择性(高达 >19:1 dr 和 99:1 er)。值得注意的是,通过微调催化剂金属中心周围手性配体的空间,实现了醛亚胺和苯乙烯的非对映发散不对称[3 + 2]环化,具有高水平的非对映选择性和对映选择性,为从同一组起始材料合成一类新型手性 1-氨基茚满衍生物的反式和顺式非对映体,该衍生物含有两个连续的立体中心。此外,还实现了醛亚胺与脂肪族α-烯烃、降冰片烯和1,3-二烯的不对称[3+2]环化。
更新日期:2023-07-28
中文翻译:

钪催化 C-H 活化通过醛亚胺与烯烃的 [3 + 2] 环化反应对映选择性合成 1-氨基茚满
多取代手性 1-氨基茚满是许多药物和生物活性分子的重要成分。因此,开发有效且选择性的手性1-氨基茚满合成方法具有重大意义和重要性。原则上,醛亚胺与烯烃通过 C-H 活化进行不对称 [3 + 2] 环化是构建手性 1-氨基茚满的原子效率最高且最直接的途径,但迄今为止这种转化仍未得到开发,可能是由于缺乏合适的催化剂。在此,我们首次报道了通过手性半夹心钪催化剂的邻-C(sp 2 )–H活化对多种芳香醛亚胺和烯烃进行对映选择性[3 + 2]环化,这为多取代手性1-氨基茚满的合成。该方案具有 100% 原子效率、广泛的官能团兼容性以及高区域选择性、非对映选择性和对映选择性(高达 >19:1 dr 和 99:1 er)。值得注意的是,通过微调催化剂金属中心周围手性配体的空间,实现了醛亚胺和苯乙烯的非对映发散不对称[3 + 2]环化,具有高水平的非对映选择性和对映选择性,为从同一组起始材料合成一类新型手性 1-氨基茚满衍生物的反式和顺式非对映体,该衍生物含有两个连续的立体中心。此外,还实现了醛亚胺与脂肪族α-烯烃、降冰片烯和1,3-二烯的不对称[3+2]环化。







































 京公网安备 11010802027423号
京公网安备 11010802027423号